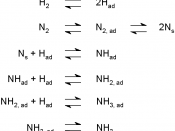At the beginning of the 20th century, the world was in need of amounts of nitrate (NaNO3), as it was feared by scientists that it was running out, and that soon a new way to cultivate crops with nitrogen was needed. Then the Haber Process was brought along. It was developed by two German men, Fritz Haber and Carl Bosch before WW1. Once discovered, the Germans kept it to themselves in an advantage of the growing need of nitrate. The actual process involved the reaction between Nitrogen (N) from the air and Hydrogen (H) derived mainly from natural gas, to create Ammonia. There have been both good and bad outcomes of the Haber Process, although nowadays I think it would be hard to fault it!
To make the product ammonia, like I said before, you need Nitrogen and Hydrogen. In order to utilise the Haber Process, you must also have; an iron catalyst to speed up the reaction, high pressure and a medium temperature at around 400/500 degrees centigrade.
The reaction between Nitrogen and Hydrogen is reversible - equilibrium. The reaction also gives off heat, also known as exothermic. The equation for this reaction is:
N2(g) + 3H2(g) 2NH3(g) + 92 kJ.
Both the Nitrogen and Hydrogen are trying to reach this Equilibrium, and so are about 50% each. But is this ok?? Le Chatelier's Principle tells us that the more you increase the temperature, the less your yield of ammonia will be, but the rate will of the reaction will be higher, because the reaction is exothermic. On the opposite side though, the lower the temperature, then the yield of ammonia will be higher, but the rate of the reaction will slow down. Therefore you need to find a balance between the two so that you can...