The atom has been suggested as the smallest complete part of matter since the time of the ancient Greek philosophers but they did not have a clear picture of the nature of the atom or any possible structure because of limitations to their scientific knowledge. Dalton was a scientist who suggested in 1810 that the principal feature of atoms is that an element is a pure substance because it contains identical atoms. This was the first time that atoms were regarded as identifiably unique because Dalton also proposed that atoms of different substances were different to each other, particularly because they had different atomic mass.
Dalton's theory lead him to also suggest that compounds consisted of different elements whose atoms had been combined together and he therefore also put forward the idea that different compounds remained consistent in the ratio of the elements involved. Dalton also suggested that chemical equations occurred when combination of atoms were rearranged.
Daltons model is shown below
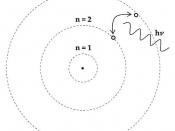
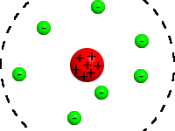
The problem with Daltons model is that it regarded atoms as units without structure and it needed further research to show that his model was incorrect. Thomson in 1904 put forward a model, which suggested that there were sub-atomic particles. Thomson suggested the atom
consists of a positively charged fluid in which negative electrons are embedded in the same way that fruit can be found throughout the ball of a pudding.
For this reason, Thomson's model is sometimes referred to as the plum pudding model. This model was incorrect in the way that he imagined that an atom was structured but the important contribution was recognising that there were negatively charged particles such as electrons, which were balanced against positively charged material.
Thomson's model is illustrated below
This model was supported by Thomson's experiment using a cathode ray,