The periodic table was an evolution in the history of chemistry. It aided scientists to predict elements that existed but not yet found. It helped people to interpret and understand the behavior and physical properties of various elements. The periodic table was developed and improved by scientists throughout the century as new discoveries were found.
In the early eighteen hundred's, Dobereiner discovered that elements that are similar in appearance and behavior also have similar atomic masses. At the same time, Dechancourtois made a cylindrical table of elements to show the reoccurrence of properties. In the eighteen six tee's Cannizro discovered weights of sixty elements that was known at the time. Afterwards, a table was arranged by Newlans and each element was given a number based on their atomic weights. Lothan Meyer and Dimity Mendeleyev were the pioneers who started the concept of organizing elements in a table based on their physical and chemical properties.
Scientists back then suggested that an element is something can not be longer broken down. Mendeleyev was a Russian and Meyer was a German. Meyer arranged fifty-seven elements known to him in the order of atomic weights and he left blank for the missing elements. He published his first period table in eighteen-seventy after he completed it two years before. He Mendeleyev predicted the chemical and physical properties of missing elements based on the trends of the periodic table. He began by writing fact about each element on paper cards. On those cards, he wrote information such as melting points, densities, colors, atomic masses, and bonding powers. Afterwards, he arranged and rearranged those cards. Many of the elements he predicted were discovered later on such as gallium, scandium, and germanium. Mendeleyev also published a book called "Principles or Chemistry". In eighteen-ninety-five, Lord Rayleigh found a new...
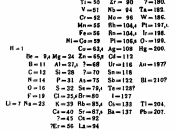

Omg! lol
sorry, its just that i had a essay question on my chemistry paper on the history of the periodtic table, lol, if i had only read this, but anyway, very good, liked it alot. Yet you could have but the essay into paragraphys but i no u did it at the start, but i looks neater, loved it, and thanks for sharing.
2 out of 3 people found this comment useful.