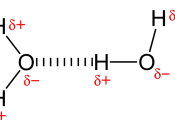
Hydrogen bonding can simply be defined as a strong type of intermolecular dipole-dipole attraction in which hydrogen is attracted to nitrogen, oxygen or fluorine. However, this does not take into account the reasons behind the attraction or why dipole-dipole attraction occurs.
Covalent molecular substances do not carry a whole charge since no atoms within the molecule lose or gain electrons unlike with Ionic or Metallic bonding where ions or, in the case of metallic bonding, cations are produced. It is known that in ionic and metallic substances, the ions are held together by the strong attraction between oppositely charged ions. However, this is not possible in the case of molecular substances since they do not carry a charge. This is partially the reason why molecular substances do not usually exist as solids, but they are able to be held together in the liquid or gaseous state by intermolecular forces known as dipole-dipole attraction.
The concept of dipole-dipole attraction involves the ability of an atom within a molecule to attract the electrons that is involved in a covalent bond. The bonding pairs of electrons will be attracted to one side of the atom greater than the other one because it is said to have greater electronegativity which is the ability of an atom to attract electrons involved in covalent bonds. Therefore atoms are either partially negative or positive.
If one side of a molecule is positive whilst the other side is negative it is known as a polar molecule where the distribution of charge is uneven or asymmetrical. The intermolecular forces between polar molecules are described as being an electrostatic force of attraction between oppositely charged ends of the molecule which is known as dipole-dipole attraction.
The strength of the dipole-dipole attraction depends on the difference of electronegativity between...