Isaac Newton discovered that when white light travels through a prism, a rainbow appears, this is called a spectrum. One is usually taught that the spectrum contains 7 colors: red, orange, yellow, green, blue, indigo and violet. But in reality there are only 6 (indigo does not exist). The colors of the spectrum actually correspond to the different wavelengths of light. When the different wavelengths travel through the prism they bend at different angles. This is a continuous spectrum. But there is also a different spectrum called the line spectrum.
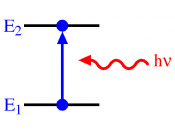
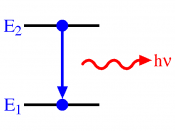
The hydrogen spectrum is a line spectrum. Lines of colored light, which represent the wavelength, are given off when atoms gain or loose energy. Light carries energy, photons. Each photon has a specific wavelength and energy quantity. When an atom gains energy, this is called excitation, the change in energy, produces light. When a high voltage is applied to hydrogen, it pulls the electrons of the atom apart and leaves behind a positive ion, leaving behind empty electron shells.
Therefore the electrons remaining can choose in which shell to go in. When they jump into a shell they loose energy. This loss of energy gives out light. The electron shells are equivalent to energy levels; each energy level have an exact frequency, and therefore a fixed energy level which therefore mean that the electron can only give off a fixed amount of energy. This is why we get the different colors. The lines in the spectrum represent the transition in energy levels. The lines of the spectrum converge as the frequency increases. The hydrogen spectrum is made up of 3 different series: the visible, ultra-violet infrared. Energy level converge because the bigger the jump the higher the frequency.
There can be two types of line spectrums, emission...