Adrian Castaneda
11/22/08
Types of Bonding Essay
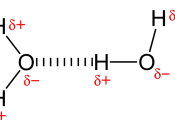
There are three types of bonds that may occur when bonding elements. They are: ionic bonds, covalent bonds, and metallic bonds. The whole objective of element bonding is to achieve stability or noble-gas form from each element. In order to do so, each element has to reach its octet or have a total of eight valence electrons. The element Hydrogen only needs two valence electrons for it to achieve stability. In case you are wondering, valence electrons are negatively charged subatomic particles that circle in the outermost cloud on an atom, and they maybe either shared, transferred, or given off to another element to achieve stability when bonding. Atoms may form single bonds, double bonds, or, sometimes, triple bonds. Triple bonds are the strongest and single bonds are the weakest. "One analogy you can use is to think about atoms as nerf balls and bonds as rubber bands.
The rubber bands act as the force which holds the balls together, as we increase the number of rubber bands the balls are squished closer together and it takes more force to pull them apart" (Bond Length-Bond Strength).
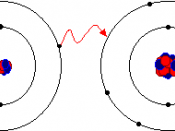
In ionic bonding, electrons are transferred from one atom to another (Carpi 2003). A real life example of an ionic bond might be lending your friend money to so they may buy lunch. An example of an ionic bond would be sodium with chlorine (Carpi 2003). Sodium, a silver colored metal, will react violently with the toxic chlorine gas, and each sodium atom will give up an electron to each chlorine atom (Carpi 2003). The result of this ionic bonding is the formation of your everyday table salt (sodium chloride). Ionic bonds have their own characteristics that make them somewhat easy to distinguish. Ionic bonded compounds have...