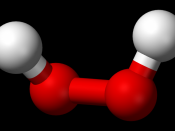
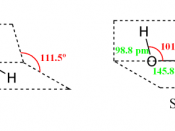
Decomposition of hydrogen peroxide
2H2O O + 2H2O
P
lanning
Aim:
Our aim is to investigate the effect that temperature has on the rate of reaction and the amount of oxygen that is produced.
Prediction:
With reference to my theory, I predict that the initial rate of reaction will increase with the temperature rise up to 50ðC where the enzyme used and released by the yeast will become denatured and wont function normally, reducing the initial rate of reaction greatly. This is explained through the kinetic theory, collision theory and the lock and key hypothesis.
Hypothesis and theory:
Catalase is an enzyme found in food such as potato. It is used for removing hydrogen peroxide from cells. Catalase speeds up the decomposition of hydrogen peroxide into water and oxygen. It is able to speed up the decomposition because of the shape of the hydrogen peroxide molecule. Enzymes are proteins that act as catalysts.
They are made in cells. A catalyst is something, which speeds up a reaction but doesn't get used up in the reaction. One can usually be used many times.
In this experiment the enzyme is the catalase in the yeast and the substrate is the hydrogen peroxide. There are several theories, which has led me to predict that as the temperature increases, the rates of reaction will increase. However, when the temperature gets above 50ðC, I think that the rate of reaction will begin to decrease. One of the theories is the collision theory. 'Chemical reactions occur when particles of the reactant collide with enough energy' (John Holman p225). If the temperature of the solution is high then there is more chance of the particles colliding. This is because if the temperature increases the molecules have more energy so there is more collision. The more energy particles...