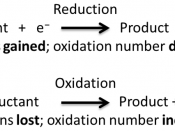
IntroductionAn electrolysis cell uses an external source of voltage to bring about a non-spontaneous redox reaction. In an electrolytic cell electricity is passed through an electrolyte and electrical energy is converted into chemical energy. Electrolysis enables neutral elements to be released from ionic compounds by discharging the ions through oxidation and reduction reactions. The power source pushes electrons towards the negative electrode where they enter the electrolyte. They are released at the positive terminal and returned to the source. The current is passed through the electrolyte by the ions as they transfer to the electrodes. Electrons always flow from anode to cathode.
AimInvestigate a factor that affects the volume of H2 at the cathode in an electrolysis reaction.
HypothesisI think that the voltage will be one factor that will affect the volume of H2. I guess that as the voltage becomes stronger the volume of H2 at the cathode will increase.
This is because how I stated in the introduction, an electrolysis cell uses an external source of voltage to bring about a redox reaction. So I think that as the voltage increases, the volume of H2 will increase.
VariableIndependent variableVoltage of the cell battery (6V, 9V, 12V)Dependant variableThe amount of H2 produced. (measure by checking the amount of electrolyte (HCl) decreased in the measuring cylinder placed on top of the anode in the electrolytic cell)Control variableTemperature of the liquids used in the experiment (room temperature)Time of the process to measure the amount of H2 produced. (use a stopwatch to keep the time constant to 5 minutes)Concentration of the electrolyte (0.25M)Volume of the electrolyte in the electrolytic cell and the measuring cylinder. (400ml)Equipment used for each trial.
MaterialCell battery (one)Beaker (one 1000ml)Measuring cylinder (one 50ml)Electrical conductors (two wires)Electrolyte (HCl 0.25M 400mlÃÂ3, 50mlÃÂ3)Electrode (copper zinc)StopwatchMethodConnect the electrical conductors to the cell battery and the electrode.
Place 400ml of HCl into a clean 1000ml beaker.
Place the wire connected electrode inside the beaker filled with HCl.
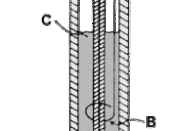
Fill the measuring cylinder full with 50ml of HCl as shown on the picture.
Place the HCl filled measuring cylinder on top of the cathode without letting air get in as shown on the picture.
Switch the cell battery on and turn the voltage to 6V. On the same time start the stopwatch.
When it is 5 minutes, observe the measuring cylinder to check and note the decrease of electrolyte in the measuring cylinder.
Repeat the experiment for 9V and 12V.
Repeat 3-5 times.
Collection of sufficient relevant dataVoltage of the cell battery (V) (ñ0.5)Amount of H2 produced (ml) (ñ0.1)Trial 1Trial 2Trial 36V2.01.82.09V3.33.23.012V5.04.84.9Process dataAverage H2 produced in 6V, 9V, 12V6V = (2.o+1.8+2.0)/3=1.9ml9V = 3.2ml12V = 4.9mlConclusionThe result was the same as what I stated in my hypothesis. As you can see from the graph, as the voltage increases, H2 is more produced. This is because an electrolysis cell uses an external source of voltage to bring about a non-spontaneous redox reaction, pumping electrons into the electrolytic cell which drives the redox reaction. As a conclusion if the voltage is increased the amount of H2 also increases.
Sourcehttp://en.wikipedia.org/wiki/Electrolysishttp://en.wikipedia.org/wiki/H2