Investigating the rate of electrolysis.
An investigation to find out what factors affect the rate of electrolysis of a solution containing copper (II) ions.
Plan
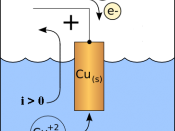
It is known that by passing a constant electric current through a copper sulphate solution the passage of ions through this solution results in copper atoms being dissolved into the solution from the anode, which has a positive charge while positive copper, ions (cations) are also being discharged at the cathode which has a negative charge. Normally anions, which have a negative charge, are discharged at the anode.
The experiment I will be carrying out is aimed to observe the amount of Copper (Cu) metal deposited during the electrolysis of Copper Sulphate solution (CuSo4) using Copper electrodes, when certain variables are changed
In this investigation I will change variables within the experiment, which will hopefully change the rate of reaction and also the deposit of copper metal at the cathode.
These variables could include:
÷ Voltage
÷ Concentration of solution/ Quantity of Solution
÷ Surface area/ Size of Electrodes
÷ Temperature
÷ Molarity/Concentration of Solution
÷ Distance between the electrodes
These variables all have a way of changing the rate of reaction.
Voltage:
Changing the voltage of the circuit would affect the rate of reaction because as Ohm's law states, As charged particles try to make their way round a circuit they encounter resistance to their flow which means that they collide with atoms in the conductor. More resistance means that more energy is needed to push the same number of electrons through part of the circuit. So by increasing the voltage more electrons will flow through the circuit, which means there would be a lot more electrons flowing in the circuit therefore there will be a lot more energy being produced. This means that...

Good, but...
Well written and laid out, but you seem to have failed to mention the molarity of your copper sulfate solution?
1 out of 1 people found this comment useful.