Investigating Some Factors Which Affect the Rate of Reaction Between Hydrochloric Acid and Sodium Thiosulphate
Background Information
Reactions can be completed at different rates. The rate of reaction is measured by observing how quickly reactants are used up or how quickly products are forming. This can be calculated in three ways:
oPrecipitation - When the products of the reaction is a precipitate, which will cloud the solution. Observing a marker through the solution and timing how long it takes for this marker to disappear will determine the rate of reaction.
oChange in Mass - Reactions which produce gas can be carried out on a precise set of scales and the decrease in mass can easily be measured.
oVolume of Gas Given Off - Using a gas syringe, the volume of gas given off during an experiment can be measured.
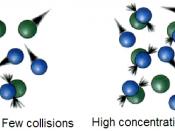
In order for particles to react, they must be colliding with each other with a sufficient impact.
This is known as the Collision Theory. To increase the rate of reaction, the number of collisions between the particles must increase. This can be done in a number of ways:
÷Temperature - Higher temperatures increase the rate of reaction, as there are more high-energy molecules. Reactions can only take place if the particles collide with enough energy. At a higher temperature, there will be more particles colliding with enough energy to make the reaction happen. This energy is known as the activation energy and is needed to break initial bonds. Raising the temperature by 10úC causes the number of high energy molecules to double. This means the rate of reaction also doubles and the time taken for the reaction to be completed is halved.
÷Agitation - This is commonly known as stirring. When a reaction mixture is undergoes vigorous movement, the particles in...