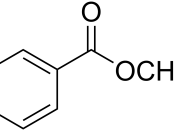
Lab 6: Electrophilic Aromatic Substitution(1) Nitration of Methyl Benzoate(2) Synthesis of 1,4-Di-t-butyl-2,5-dimethoxybenzene byFriedel-Crafts Alkylation of 1,4-DimethoxybenzenePurpose1)To carry out the nitration of methyl benzoate, and then identify the major product formed (position at which nitro-group substitution takes place) by thin-layer chromatography (TLC), the percent yield and the melting point range.
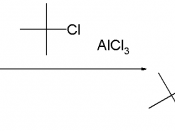
2)To synthesize 1,4-Di-t-butyl-2,5-dimethoxybenzene by Friedel-Crafts Alkylation of 1,4-Dimethoxybenzene, and then determine the percent yield and melting point range.
Procedure*Please refer to the lab handout 6 and Macroscale and Microscale Organic Experiments (Williamson, 2003).
* Part II of the experiment (Synthesis of 1,4-Di-t-butyl-2,5-dimethoxybenzene by Friedel-Crafts Alkylation of 1,4-Dimethoxybenzene) was carried out by Ashley and me. Part I (nitration of methyl benzoate) was carried out by Jenny.
Physical Quantity TableType of substanceMolecular FormulaMolecular Weight (g/mol)Density(g/cm3)M.P.(oC)B.P.(oC)Methyl benzoateC8H8O2136.161.094-15198-200Methyl 2-nitrobenzoateC8H7NO4181.141.289-13104Methyl 3-nitrobenzoateC8H7NO4181.14-78-80289Methyl 3,5-dinitrobenzoateC8H6N2O6226.14544-106-109-Methyl 4-nitrobenzoateC8H7NO4181.15-94-96-1,4-DimethoxybenzeneC8H10O2138.161.0555-582131,4-Di-t-butyl-2,5-dimethoxybenzeneC16H26O2250.37-104-105-2-methyl-2-propanolC4H10O74.12-25.482.4Hazard Concentrated sulfuric acid and nitric acid are highly corrosive.
ObservationPart II Friedel-Crafts AlkylationThe concentrated sulfuric acid used was yellow.
1,4-Dimethoxybenzene was in white crystal form. The t-butyl alcohol solidified in room temperature, so it took a while to heat it up and return to liquid form. After concentrated sulfuric acid was added to the t-butyl alcohol, acetic acid and 1,4-Dimethoxybenzene mixture, the solution became light brown in color. After warming for a while, white precipitate could be observed at the bottom of the tube. After water was added, the white precipitate dissolved and the solution turned milky. The crystals obtained after recrystallization are in form of large slides. They are grinded into powdery or smaller granules for melting point test.
DataPart IMass of methyl benzoate = 0.30gMass of recrystallized product = 0.28436gMelting Point Range of nitration product = 75 oC - 83 oCChemical CompoundsDistance from B to spots (cm)Distance from B to SF(cm)Distance from B to spots (cm)/Distance from B to SF (cm)Retention FactorRfMethyl benzoate1.684.501.68/4.500.37(ortho) Methyl...