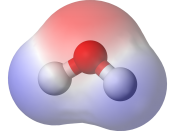
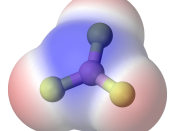
In this lab report we had to determine the most common type of bond between two atoms is a covalent bond. A covalent bond is formed when two atoms share a pair of electrons. If both atoms have the same electro negativity, or tendency to attract electrons, the bond is non-polar covalent. When atoms have different electro negativities, the electrons are attracted to the atom with the higher electro negativity. The bond that forms is polar covalent.
Molecules made up of covalently bonded atoms may themselves be polar or non-polar. If the polar bonds are symmetrical around the central atom, the bonds offset each other and the molecule is non-polar. If the polar bonds are not symmetrical, the electrons will be pulled to one end of the molecule and the molecule will be polar.
Many physical properties of matter are the result of the shape and polarity of molecules.
In this experiment, you will build models of molecules and predict their polarity based on their shape.
In this lab we he had to construct models of molecules, determine molecular shapes, and predict polarity of molecules. First we had to build a model for each of the following molecules. I then drew all the shapes for each molecule.
N2 HCOOH HBr C3H6 H2O CH4 NH3 C2H5Cl CH3NH2 C4H8 (ring) CO2 C2H60 (two structures) Formula 3-D Diagram Shape Polarity N2 Linear non-polar HBr Linear non-polar H2O polar NH3 Tripod non-polar CH3NH2 Double tetrahedron polar CO2 Bent non-polar HCOOH tetrahedron polar C3H6 non-polar CH4 tetrahedron non-polar C2H5Cl Double tetrahedron polar C4H8 non-polar (ring) C2H6O irregular tetrahedron polar a. C2H6O Double tetrahedron non-polar b. How can molecular polarity be determined on the basis of molecular shape? If the shape is symmetrical then the molecule is non-polar. If it is a symmetrical itâ??s polar.