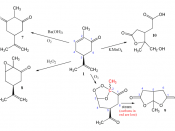
Optical Activity of Carvones
Kyle Peterson
Chem. 243a
Matt Judd, Sec. 25
Date Performed: 10/29/03
Abstract: The objective of this experiment is to use Gas Chromatography to distinguish between two enantiomers of carvone from caraway oil and spearment oil and to find the 2 carvone's optical activity as well as percent carvone in spearment and caraway oil. It was found that S-carvone had an optical activity of 0.0047 and R-carvone had an optical activity of 0.516 and that spearment oil is 59% carvone and caraway oil is 100% carvone.
Backround:
Gas Chromatography separates organic samples much in the same way as column chromatography. The only differences are that it uses a moving gas phase and a stationary liquid phase, and that the temperature of the gas system can be controlled. In a gas chromatograph the sample is shot in with a syringe and is immediately vaporized in a heated injection chamber.
It is then introduced to a moving stream of gas called the carrier gas which sweeps the vaporized sample into a column filled with particles filled with liquid adsorbent. This column is usually filled with liquid that has a low vapor pressure and high boiling and is called the stationary phase. This phase is also usually coated onto a support material very evenly and packed into a tubing apparatus as evenly as possible and placed in the temperature controlled oven. When organic solutions are passed through the tubing van der Waals forces attract the nonpolar molecules especially if they have large molecular weights. Polar molecules can be attracted in many ways. Interactions include salt formation, coordination, hydrogen bonding, and even dipole-dipole. Through these interactions the molecules in the vaporized sample will separate accordingly. Finally at the end where the gases come out is a detector which generates a signal...