Fatima Mammadova Afagi
M5A
Chemistry
Mr. Brotherton
One World Essay
What limits the way that batteries can change the way we live?
Battery is a device, which consists of one or more electrochemical cells, which converts stored chemical energy into electrical energy. Batteries are used everywhere in our daily life. We use them in technology, like mobile phones, computers, music players and etc. We use them for medical reasons, like wheelchairs, defibrillator, pace makers, hearing aids and many others. Batteries have solver an enormous amount of problems. For instance, without batteries there wouldn't be mobile phones and computers.
Batteries work by reduction and oxidation reaction. Battery is a device, which consists of one or more cells. Each cell consists of at least two half-cells: a reduction cell and an oxidation cell. Chemical reactions in the two half-cells provide the energy for the battery. (Wikipedia) The reason why chemical reactions happen is that one element is more reactive than the other.
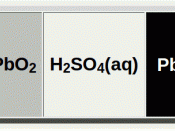
Oxidation is a loss of electrons and reduction is a gain of electrons. Each cell consists of two metal electrodes (cathode (-) and anode (+)) and at least one electrolyte solution (solution which can conduct electricity). Oxidation occurs at the anode and reduction occurs at the cathode. (Gary L. Bernard) Oxidation either removes negative ions or produces positive ions to the solution at the anode. Reduction does the opposite; it either removes positive ions or produces negative ions in the solution at the cathode. (Bryant, Charles W) In order for electrons to move freely through the wire, there must be a path for electrons. Putting the two solutions together could make this path, but that makes the energy run out too quickly. (Science)
The first battery was invented in 1800 by a scientist- Alessandro Volta. In 1780, Volta saw his...