The conservation of mass
AIM
The aim of this experiment is to show that mass is conserved during a chemical reaction.
The Law of Conservation of Mass, established in 1789 by French Chemist Antoine Lavoisier, states that mass is neither created nor destroyed in any ordinary chemical reaction. Or more simply, the mass of products by a chemical reaction is always equal to the mass of the reactants.
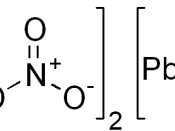
The reaction between potassium iodide and lead (II) nitrate under room temperature and atmospheric pressure results with a yellow precipitate. This reaction can be expressed by the equation: 2KI(aq) + Pb(NO3)2(aq) ( PbI2(s) + 2KNO3(aq)
HYPOTHESIS
The mass before and after a chemical reaction will be the same as mass is conserved.
INDEPENDENT VARIABLE:
The mass before the reaction
(By measuring the mass of the reactants before reaction this independent variable can be controlled)
DEPENDENT VARIABLE:
The mass after the reaction
(By controlling the independent variable this dependent variable can be controlled)
CONTROLLED VARIABLES:
VARIABLE |
Digital balance |
Proportion of chemicals |
Beakers |
Reaction time |
MATERIALS
NAME | SIZE/CONC. | AMOUNT | PRECISION |
Beaker | 100 mL | 2 | Irrelevant |
Measuring cylinder | 25.0 mL | 2 | Irrelevant |
Access to a digital balance | ----------- | 1 | ñ0.001g |
Potassium iodide solution | 0.5 mol/dm3 | 100 mL | Irrelevant |
Lead (II) nitrate solution | 0.5 mol/dm3 | 100 mL | Irrelevant |
Glass rod | 20 cm | 1 | Irrelevant |
METHOD
Wear safety glasses, apron and covered shoes before setting up any equipment.
Measure out 20 mL of potassium iodide with a measuring cylinder and pour into a 100 mL beaker.
Measure out 20 mL of lead nitrate with another measuring cylinder and pour into another 100 mL beaker.
Zero the balance and put both beakers together on the balance (balance is big enough for two beakers). Weigh their total mass accurately.
Record the mass obtained including uncertainties.
Carefully pour the lead (II) nitrate solution into the 100 mL beaker containing the potassium iodide.
Record the observation during the reaction.
Use a glass rod to carefully stir the mixture in order to make sure the reaction is completed. Re-zero the balance. Place both beakers back on balance and weigh their total mass.
Record the mass obtained including uncertainties.
When finished, pour the contents of the beakers into the laboratory sink.
Rinse the beakers and the measuring cylinders under tap water.
Dry the beakers and the measuring cylinders.
Repeat steps 2-12 another four times to maintain accurate results.
Result
Trials | Mass before weighing(ñ0.001g) | Weight after weighing(ñ0.001g) |
1 | 138.805g | 138.785g |
2 | 139.007g | 138.997g |
3 | 138.794g | 138.776g |
4 | 138.638g | 138.609g |
5 | 138.798g | 138.770g |
Qualitative data:
Yellow precipitate was produced.
There were some spillages of the product.
Processed data
Trials | Mass difference ñ 0.002g | Mass difference express as percentage | Random Error |
1 | 0.020 | 0.014% | 0.0014% |
2 | 0.010 | 0.0072% | 0.0014% |
3 | 0.018 | 0.0013% | 0.0014% |
4 | 0.029 | 0.021% | 0.0014% |
5 | 0.028 | 0.020% | 0.0014% |
Average | 0.021 | 0.013% | 0.0014% |
Conclusion
The mass of both the beakers and their contents before the reaction was not the same as the mass after the reaction had taken place. The processed data table reveals that the mass difference before weighing and after weighing is 0.021g which is greater than the acceptable error range, ñ0.002g, indicating that the mass is not conserved and hypothesis is not supported. It is suggested that this experiment relatively inefficient in terms of investigating the conservation of mass in a chemical reaction. The calculated random error was 0.0014%, the mass of the products from this reaction is not within the limits of random experimental error. The percentage discrepancy shown by the result is 0.013%. Since mass is conserved in a chemical reaction, this indicates the discrepancies in respect to systematic errors or mistakes.
Evaluation
Humidity (that was not identified at the beginning) has an effect on the experiment. However during the experiment humidity, pressure and temperature are assumed to be constant.
From the processed data table it can be seen that trial 1, 3, 4 and 5 have similar results hence they are reliable. On the other hand, trial 2 has a relatively low mass difference (0.010g) which could be an unreliable datum.
Errors | Improvements |
Random Errors This experiment includes random errors such as fluctuation of the electronic balance. The processed data table has displayed all the uncertainty values. Additional random errors that may have been overlooked or underestimated may include: Extent of fluctuation of the balance The readings on the balance showed an extent of fluctuation of ñ0.002g, which is over ñ0.001g. Reading the electronic scales Electronic scales were not read consistently. This resulted in a false estimation of the true value and data would be recorded imprecisely. Consideration of these additional random errors accounted for a doubling of the uncertainty to 0.0028% but this is still a diminutive quantity of 0.013% discrepancy. | Random Errors In order to reduce and minimise the Random Errors listed above, the following precautions can be taken: Extent of fluctuation of the balance Observe the display for a longer period of time and record the correct uncertainty until there is a stable extent of fluctuating data. Reading the electronic scales Since this error cannot be avoided but can only be minimised, several trials of same original data reading should be taken and recorded. The average reading should be calculated in order to increase the precision of the result. For example having the mass 138.805g and weighing the mass of the beakers with their content afterwards for 3 or 4 times and their average data will be more precise. |
Systematic errors Systematic that occurred during the experiment are listed below: Heat Released during the reaction In this experiment the reaction exhibits a relatively high energy that decreases to an internal energy. This reduction in energy is exhibited as heat release in the form of water vapour to the surroundings and is called an exothermic process. In this experiment open beakers were used to measure the mass of the products of the reaction. Gas given off during the reaction escaped into the atmosphere and hence was not measured by the electronic scale. This will effect a reduction in the mass loss evident in the experiment Electronic balance inaccuracy and inconsistency Faulty calibration of electronic balance had led to inaccurate results. The true mass could be higher or lower than the display on the balance. Residue on the stirring rod After pouring the Pb(NO3)2(aq) solution into the beaker containing KI(aq) solution, a glass rod was used to stir the solution to ensure the completion of the reaction. However, after the glass rod was taken out of the solution, there were small amounts of residue of the PbI2(s) precipitate stuck onto the rod and they could not be cleaned or removed. This resulted in the mass loss of PbI2 precipitate, causing the actual total mass after the reaction to be slightly lower than the theoretical mass. By observation, the residue of precipitate left on the rod is estimated 0.005 g and thus an approximately 0.004% overall relative error. | Systematic errors The Systematic Errors can be rectified in the following way: Heat Release during the reaction To minimise the error caused by releasing energy during the reaction, a lid should be immediately placed on the beaker after the reaction starts. This will seal the beaker and will help to prevent the escape of water vapour produced by the reaction. and will reduce the total mass loss. Consequently error caused by heat release will be considerably reduced. Electronic balance inaccuracy and inconsistency The exact inaccuracy and inconsistency could be found by weighing an object having a mass that is already known (e.g. a pen) on the digital scale used in this experiment. Weigh it for several times and record the readings on the scale. Compare the data with the real value so the difference between the actual value and the readings on the scale will be determined. This difference can be eliminated by subtracting it from the result from this experiment. This experiment can also be enhanced by weighing the known object using other digital balances. By comparing their values with the real value a more precise balance could be utilised in sequent experiment. Residue on the stirring rod Using stirring rod reduced the efficiency of the experiment as there were some residue of precipitate left on the stirring rod and they could not be removed. Alternatively, shaking the beaker gently would be a better way to ensure the reaction is completed because this avoids any direct contact between the experiment apparatus (other than the beaker) and the products. Also, weighing the rod with the beakers before and after reaction could avoid the errors generated by the stirring rod. Therefore, total mass loss caused by loss of the precipitate could be prevented. |
Mistakes Mistakes occurred were recorded in the Qualitative Table. Most listed can be associated with a reduction in mass loss. Lead (II) Nitrate was not thoroughly transferred into the beaker. Spillage of the solution while transferring for weighing will affect the result an estimate of 0.005g or 0.004% of errors taking place(by observing the spillage and roughly estimating its mass). | Mistakes To avoid the Mistakes in future experiments the following modifications can be made: A dropper should be used for transferring the solutions in this experiment so that the Lead (II) Nitrate and Potassium Iodide solutions can be transferred from their respective measuring cylinders to the beakers more safely and conveniently and without spilling. Take time to do the experiment so as to prevent spillage while transferring the beakers (after reaction) for weighing. |
Reference list
Ed Durnford . 1999. The Law of Conservation of Mass. [ONLINE] Available at: http://www.mi.mun.ca/users/edurnfor/1100/atomic%20structure/tsld004.htm. [Accessed 10 April 2013].
2013. quantitative chemistry: heat and chemical reactions. [ONLINE] Available at: http://www.iun.edu/~cpanhd/C101webnotes/quantchem/rxnheat.html. [Accessed 11 April 2013].
2013. Conservation of Mass Lab. [ONLINE] Available at: http://raytedder.tripod.com/chemistrymaterialsfromraytedder/id27.html. [Accessed 11 April 2013].
2013. report format: experiment #3 conservation of matter . [ONLINE] Available at: http://www.google.com/url?sa=t&rct=j&q=mass+conservation+lab+report&source=web&cd=34&ved=0CDsQFjADOB4&url=https%3A%2F%2Fhwchemistry.wikispaces.com%2Ffile%2Fview%2FREPORT%2BFORMAT%2BLAB%2B%25233.doc&ei=wz-IUcPYPPCSiQeLvoDACg&usg=AFQjCNHJq-4qPwtSlRZbFZUtEfrD98TDyQ&cad=rja. [Accessed 11 April 2013].
2013. Lavoisier and the Law of Conservation of Mass. [ONLINE] Available at: http://www.uta.edu/faculty/sawasthi/Lecture%20Notes%20Chem1451/Law%20of%20Conservation%20of%20Mass.htm. [Accessed 11 April 2013].
mh99320. (2010). High School Chemistry Lab - Law of Conservation of Mass. [Online Video]. 01 June. Available from: http://www.youtube.com/watch?v=WxsR2MOuIG0.[Accessed: 11 April 2013].