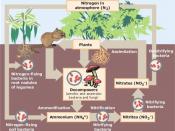
Nitrogen is essential for all life on this planet, but most of it is in the air, making up about 78% of the earths atmosphere. The growth of all organisms depends on the availability of mineral nutrients, and none is more important than nitrogen, which is required in large amounts as an essential component of proteins, nucleic acids and other cellular constituents. There is an abundant supply of nitrogen in the earth's atmosphere in the form of N2 gas. However, N2 is unavailable for use by most organisms because there is a triple bond between the two nitrogen atoms, making the molecule almost static. In order for nitrogen to be used for growth it must be "fixed" (combined) in the form of ammonium (NH4) or nitrate (NO3) ions. The weathering of rocks releases these ions so slowly that it has a neglible effect on the availability of fixed nitrogen.
So, nitrogen is often the limiting factor for growth and biomass production in all environments where there is suitable climate and availability of water to support life.
Micro-organisms have a central role in almost all aspects of nitrogen availability and therefore for life support on earth. Some bacteria can convert N2 into ammonia by the process termed nitrogen fixation; these bacteria are either free- living or form symbiotic associations with plants or other organisms (such as termites and protozoa). Other bacteria bring about transformations of ammonia to nitrate, and of nitrate to N2 or other nitrogen gases. Many bacteria and fungi degrade organic matter, releasing fixed nitrogen for reuse by other organisms. All of these processes contribute to the nitrogen cycle.
The nitrogen molecules N2 is inert. To break it apart so that its atoms can combine with other atoms requires the input of substantial amounts of energy. Three...