OSmosis is the movement of water through a weaker concentration to a stronger dilute concentration, Evaluation
Overall I think that the investigation was quite successful because it was a reasonably easy experiment to perform and get positive results from if done correctly. From the experiment I ended up with a fairly good set of results and I was able to use my results to write up a detailed page of results and evidence, and a positive conclusion. These results also allowed me to confirm the set of results with my prediction.
I believe that my results were of a good quality and that there were only a few unexpected results from my experiment. I think that they were of a good quality because when you look at my proper graph most of the concentrations on the graph let off less gas as the concentrations get weaker.
The results that I didn't expect were that on the graph the 0.9m concentration finished on 89cm3 of gas after 180seconds but the 1.0m concentration (pure acid) finished on 80cm3 of gas which is 9cm3 below. I am not sure why this happened because I predicted that the rate of reaction will decrease as the concentration gets weaker. I think this must have happened because the magnesium may have reacted more in this concentration. The same happened with the 0.6m concentration and the 0.7m because the 0.6m concentration finished higher than the 0.7m. It was only 3cm3 high so it wasn't as unusual as the 0.9m and 1.0m result. This kind of result might have happened because maybe I made the concentration with maybe a tiny extra bit of acid or maybe the magnesium ribbon just reacted more with that concentration. Another very unusual pattern I saw when...

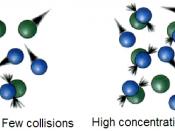
OSmosis
is from a high to low consitration, throught a simi permeable membrane.
But the rest is good, it is quite a hard topic to be evaluted on, well done!
2 out of 2 people found this comment useful.