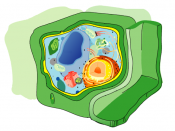
Osmosis is the diffusion of free water molecules from a region of high concentration to a region of low concentration across a partially permeable membrane. Osmosis is complete when all of the water molecules have been evenly spread out and can take place either in plant cells or animal cells, so long as a partially permeable membrane is present. Osmosis can also be conducted in the visking tubing experiment, where the visking tubing is an artificial permeable membrane. So how does Osmosis take place? When you put an animal or plant cell into liquid containing water, one of three things can happen. In the 1st option, if the medium surrounding has a higher water concentration than the cell (a very dilute solution) the cell will gain water by osmosis. Water molecules are free to pass across the cell membrane in both directions, but more water will come into the cell than leave, the net result is that water enters the cell; hence, the cell is likely to swell up.
In a plant cell, there are many structures, which include a cellulose cell wall. This outer structure gives the cell a fixed shape and can resist changes in pressure inside the cell. When the cell takes up water by osmosis and start to swell, the cell wall prevents it from bursting. Plant cells become 'turgid' when they are put in dilute solutions, which mean that it becomes swollen and hard. The pressure inside the cell rises, and eventually the internal pressure of the cell is so high that no more water can enter the cell. Turgidity is very important to plants because this is what makes the green parts of the plant 'stand up' into the sunlight. However, when animal cells are placed in sugar solutions things may be rather...