Computer
2
Computer 2
Freezing and Melting of Water
Freezing and Melting of Water
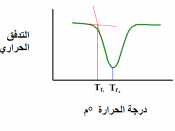
Freezing temperature, the temperature at which a substance turns from liquid to solid, and melting temperature, the temperature at which a substance turns from a solid to a liquid, are characteristic physical properties. In this experiment, the cooling and warming behavior of a familiar substance, water, will be investigated. By examining graphs of the data, the freezing and melting temperatures of water will be determined and compared.
OBJECTIVES
In this experiment, you will
Collect temperature data during the freezing and melting of water.
Analyze graphs to determine the freezing and melting temperatures of water.
Determine the relationship between the freezing and melting temperatures of water.
Figure 1
MATERIALS
computer | 400 mL beaker |
Vernier computer interface | water |
Logger Pro | 10 mL graduated cylinder |
Temperature Probe | ice |
ring stand | salt |
utility clamp | stirring rod |
test tube |
PROCEDURE
Part I: Freezing 1. Fill a 400 mL beaker 1/3 full with ice, then add 100 mL of water. 2. Put 5 mL of water into a test tube and use a utility clamp to fasten the test tube to a ring stand. The test tube should be clamped above the water bath. Place a Temperature Probe into the water inside the test tube. 3. Connect the probe to the computer interface. Prepare the computer for data collection by opening the file "02 Freeze Melt Water" from the Chemistry with Vernier folder of Logger Pro. 4. When everything is ready, click 5. Soon after lowering the test tube, add 5 spoons of salt to the beaker and stir with a stirring rod. Continue to stir the ice-water bath during Part I. Important: Stir enough to dissolve the... |