Carter French
Ms. Grand
Chemistry 1
Due 9/30/14
Periodic Element Justification Research Project
The element hydrogen, has been correctly placed on the periodic table in large part because its ionization energy, electronegativity, and Valence electrons align with general periodic trends.
In 1766, the element Hydrogen was officially recognized as a distinct element largely due to the research done by Henry Cavendish. Hydrogen was the first element to form in the universe. All other elements formed from hydrogen. Hydrogen is the most abundant element in the visible universe. Hydrogen is the first element in the periodic table because of its one proton and one electron.
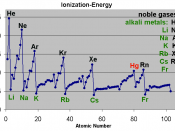
Ionization energy is the exact quantity of energy that it takes to remove a valence electron or an electron on the farthest shell. Ionization energy increases bottom to top because the number of valence electron increases bottom to top so it takes less energy to remove less electrons on the top than the bottom.
Hydrogen has an ionization energy of 13.598 EV and the element lithium is bellow hydrogen with an ionization energy of 5.39 EV. This data corresponds with the general trend of ionization energy increases bottom to top.
Electronegativity is the measure of the tendency of an atom to attract a valence electron. The general trend for electronegativity is the increase from bottom to top. This is one of the least clear trends on the periodic table because of the nonstandard way of measuring electronegativity. Hydrogen has a 2.1 on the Pauling scale for electronegativity. The element bellow hydrogen is lithium which has 1 on the Pauling scale. This makes sense because electronegativity increases bottom to top.
Valence electrons are the electrons on the farthest shell of the atom. They are also the driving force between all chemical reactions. The general trend for...