�PAGE � �PAGE �5�
Preparation and Distillation of Cyclohexane
Introduction
Green chemistry techniques use some or all of the twelve basic principles that are recognized by the Environmental Protection Agency as environmentally responsible (1). The first principle to prevent waste is perhaps the most important - if there is no waste to clean or treat the impact on the environment is essentially absent. Principle five advocates the use of catalysts and not stoichiometric reagents. Since catalysts are used in small amounts and recycled they are much more efficient to use rather than an excess of reagent that only works on one reaction.
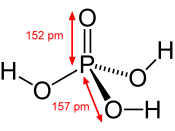
In this project, phosphoric acid (H3PO4) is used as a catalyst to produce cyclohexene rather than the less green alternative - sulfuric acid (H2SO4) (2). Cyclohexene occurs in coal tar and is prepared in the lab by dehydration of cyclohexanol at high temperatures (3). The acid is used in the preparation as the dehydrating agent.
Phosphoric acid has a pH of 1.5 instead of the 1.2 pH that sulfuric acid, making it safer and a greener alternative. While the higher pH makes H3PO4 safer than the H2SO4, it is possible to neutralize most acids with their corresponding base to become a less harmful aqueous solution.
In addition, it is possible to make any chemistry lab exercise, organic or otherwise, a greener project by actively monitoring waste and practicing stringent safety precautions. By accurately measuring the amounts of reagents needed excess need not be disposed of in the first place. Keeping the lab area clean, orderly, and safe is another way to protect the environment. A neat area will help to prevent accidents and spills.
Figure 1: Traditional mechanism for the synthesis of cyclohexene using sulfuric acid
http://web.fccj.org/~smilczan/ten/CYCLOH.pdf
Prior Work
There is a revolution to use more...