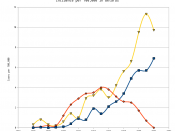
Radioactive Iodine and its Effects on the Thyroid
A Review of Literature
Stevens
The element Iodine has a multitude of properties: from cleaning and disinfecting to the detection and cure of diseases. Despite these exceptional properties, it has the potential to do harm. The purpose of this review of literature is to investigate the effects of radioactive Iodine on the thyroid gland. Investigated information on this matter includes atomic properties of the isotope and its effects on the thyroid gland.
Iodine is a nonmetallic element within the Halogen group. In solid form, it appears soft with a bluish black hue. There are over thirty known isotopes of Iodine, but the most common of the isotopes are Iodine-129 and Iodine-131. Since this review of literature focuses towards nuclear fallouts of Iodine, I-131 will be the main isotope of study. According to the Environmental Protection Agency, "Radioactive iodine has the same qualitative properties as stable iodine" (EPA); however, I-131 has a half-life of eight days and emits strong beta radiation, but in the instances of nuclear fallouts, it can emit gamma radiation.
Radiation emission comes through the forms of alpha (ÃÂ), beta (ò), and gamma (ã) decay, with alpha emitting the largest and least penetrating particles in the form of H2+ ions. Beta decay is in the form of electrons and their counterparts, positrons. These particles can penetrate the skin and upper flesh. Gamma radiation is the strongest form of radioactive decay. It is emitted in electromagnetic rays of photons. Gamma rays can completely penetrate humans with ease and can only be stopped with a heavy metal shield (normally lead) many centimeters thick.
One prominent property of I-131 its ability to sublimate, or skip the liquid state and go through a phase change from a solid straight to a gas. This makes...