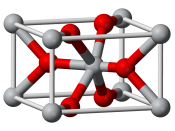
Rutile Rutile, TiO2 In the rutile structure, TiO2 has distorted TiO6 octahedra that form columns by sharing edges, resulting in Co-ordination numbers of 6 and 3 for titanium and oxygen, respectively. Adjacent columns are connected by sharing corners of octahedra. The oxide ions have three nearest-neighbour titanium ions in a planar configuration, one at a slightly greater distance than the other two.
The unit cell has titanium ions at the corners and in the body centre, two oxygens in opposite quadrants of the bottom face, two oxygens directly above the first two in the top face, and two oxygens in the plane with the body-centred titanium forming the final two positions of the oxide octahedron around the centre titanium.
Of all white pigments, TiO2 is now the most widely used. Well uses are as a surface coating on paper (25%) and as a filler in rubber and plastics (15%).
The value of TiO2 as a pigment is due to its exceptionally high refractive index in the visible region of the spectrum. Thus although large crystals are transparent, fine particles scatter light so strongly that they can be used to produce films of high opacity.
In the manufacture of TiO2 either the anatase (the other structural form of TiO2) or the rutile form is produced depending on modifications in the process employed. Because of its slightly higher refractive index, rutile has a somewhat greater opacity and most of the TiO2 currently produced is in this form.
In addition to these optical properties, TiO2 is chemically inert which is why it displaced "white lead", 2PbCO3.Pb(OH)2: in industrial atmospheres this formed PbS (black) during the production of or weathering of the paint and was also a toxic hazard.
Unfortunately the naturally occurring forms of TiO2 are invariably coloured, sometimes intensely, by impurities, and expensive processing is required to produce pigments of acceptable quality. The two main processes in use are the sulfate process and the chloride process, which account for approx. 70% and 30% respectively of the total world production. The principal reactions of the chloride process are: 2TiO2 + 3C + 4Cl2 "à ¾_ 2TiCl4 + CO2 + 2CO and then TiCl4 + O2 "à ¾_ TiO2 + 2Cl2 The physical properties of the base pigments produced from both processes are further improved by slurrying in water and selectively precipitating on the finely divided particles a surface coating of SiO2, Al2O3, or TiO2 itself.
The rutile structure is also found for MgF2, ZnF2, and some transition metal fluorides. Compounds that contain larger metal ions adopt the fluorite structure with Co-ordination numbers of 8 and 4.
Uses of Rutile Titanium Rutile (titanium oxide) and Ilmenite (titanium/iron oxide) are the chief minerals recovered from "mineral sands" mines, of which several are still operating along the NSW north coast. The abolition of mining within conservation reservations rather than declining mineral reserves has seen most of the NSW operations close and most Australian production now comes from Western Australia in the vicinity of Bunbury and Eneabba. Considerable reserves exist in Far Western NSW and in the Wimmera district of Victoria, but are only marginally economic at the present time.
Several "artificial rutile" plants have been constructed in Australia, which extract much of the iron from ilmenite, thus raising its value and suitability for many applications. Rutile is both a source of the metal titanium and has a wide range of uses in its "as mined" state. The most widespread use of rutile is as a white pigment or opacifier, which resists yellowing and breakdown by sunlight. It is chemically inert and thus can be safety used as a pigment in foods. Uses of rutile are:- "à ¾h As a pigment in paints, paper and plastics "à ¾h As an inert pigment in foodstuffs (eg. Smarties, Jaffas) "à ¾h Fluxes for welding electrodes "à ¾h SP 15+ sunscreens Titanium metal is extremely strong for its weight and maintains its strength over a wide temperature range. It is also highly resistant to corrosion and because of this, can be used as prostheses without risk of rejection by the human body. Some uses are:- "à ¾h Artificial hip, knee and shoulder joints "à ¾h Heart pacemakers "à ¾h Turbine blades for jet engines and motor vehicle turbochargers "à ¾h Aircraft and spacecraft structural components Rutile Images and structures Rutile