Silicon
Silicon the most common used element on earth was discovered I 1824, by Jons Jacob Berzelius, a Swedish chemist. Berzelius discovered this element by heating chips of potassium in a silica container and then carefully washing away the residual by-products. The atomic number is 14, atomic weight: 28.086, melting point: 1687K (1414ðC) or 2577ðF), boiling point: 3538k (3265ðC or 5909ðF), density: 2.3296 grams per cubic centimeter, and the phase at room temperature is solid. Silicon gets its name from the Latin word silex. The symbol for silicon is Si. Silicon can be found in group 14 (or IV) of the periodic table.
Silicon is prepared by a brown amorphous powder or as a gray-black crystal. Silicon is made by heating silica, or silicon dioxide (SiO2), with reducing agent, such as carbon or magnesium, in an electric furnace. Silicon dissolves in sodium hydroxide, forming sodium silicate and hydrogen gas.
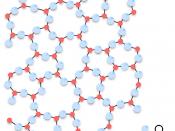
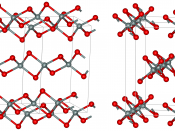
At ordinary temperatures silicon is impervious to air, but high temperatures it reacts with oxygen, forming a layer of silica that does not react further. At high temperatures it also reacts with nitrogen and chlorine to form silicon nitride and silicon chloride, respectively. Silicon constitutes about 28 percent of the earth's crust. It does not occur in the free, element state, but is found in the form of silicon dioxide and in the form of complex silicates.
Silicon containing minerals constitute nearly 40 percent of all common minerals, including more than 90 percent of igneous-rock-forming minerals. The mineral quartz, varieties are the naturally occurring crystal forms of silica. Two allotropes of silicon exist at room temperature: amorphous and crystalline. Amorphous appears as a brown powder white crystalline silicon that has a metallic luster and a grayish color. Single crystals of crystalline silicon can be grown with a process known as...