Silicon Breast Implants In the mid 20th century when Silicones were initially developed by Dow Corning, they were though to be completely inert - not reacting to any chemicals in the body. Silicones are very stable at high temperature, which allows them to be easily sterilized and used during surgery or as implants opening the possibility of a myriad of medical uses.
During the early years, animal studies showed that a whole series of familiar synthetic materials, nylon, Teflonî, Mylarî, polyester, silicone and polyethylene, could be implanted over a long period of time with minimal irritation or other physical response. The excellent implant behavior of a number of materials fueled the thinking that most synthetic polymers would be safe.
Initial small scale manufacturing of breast implants began in the early 1960's , used primarily after mastectomies.
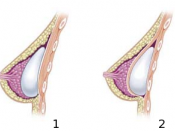
During the 70's Dow Corning made changes to the sack, eliminating a seam and making the material thinner.
The first lawsuit based upon implants rupturing came in 1977. Large scale manufacturing proceeded by General Electric in the late 1970's..
The oversimplified manufacturing process looks something like: ÷ Buy some HCl and methyl chloride.
÷ Mix Si and Cu, heat the mixture and flow the two gases, HCl and CH3Cl over the Si and Cu catalyst.
÷ Take the product (CH3)2SiCl2 (9), and react it with water.
Collect the methylsilicone polymer, form it or encase the gel form in plastic for use as implants.
Silicone is not used only for implants, it has been used for hundreds if not thousands of products ranging from gaskets, lubricants, and adhesives to a wide variety of medical and cosmetic uses including of course silicone breast implants.
In 1999 the Institute for Medicine released a study on the hazards of implants, concluding that "although silicone breast implants may be responsible for localized problems such as hardening or scarring of breast tissue, implants do not cause any major diseases such as lupus or rheumatoid arthritis." The following year, experts for the National Cancer Institute and the National Institutes of Health, prompted by a high incidence of cancer among women with breast implants, convened a symposium on "immunology of silicone." Experts at the conference concluded that "exposure to silicone breast implants sets into motion significant and long-lasting diseases. When silicone gel leaks from a ruptured implant, it poisons the system, triggering immune responses that set the body against itself." On June 21 2000, a 45-year-old woman, went to her plastic surgeon after learning that her breast implants had ruptured. For 10 years she has been suffering from the symptoms of silicone poisoning, which-include dryness of the eyes, nose and mouth; headaches, numbness and tingling in the limbs; memory loss; nausea; and arthritic conditions. Diagnosed with lupus, she is now unable to work and permanently disabled.
The debate rages to this day about the safety or lack thereof of silicone breast implants. Properly manufactured, implanted and monitored, the risk of serious disease is very low, although hardening of tissue is common. Factor in the possibility of leakage and the risk of disease is much higher.