Objectives of Experiment
The objectives of this experiment are:
1. To determine the solubility of a solute at a different temperature
2. To state the differences between a saturated and an ordinary solution
Theory and Background
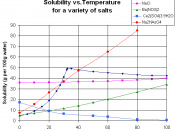
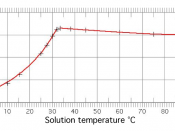
Solubility is a measure of how easily one substance can dissolve in another.
The more soluble a substance is in water, for example, the more of it you can dissolve in
water before it starts to pile up at the bottom. When more of a substance can still be
dissolved in water, we say the water is unsaturated. When no more of the substance will
dissolve, we say the water has become saturated. Ionic solids (or salts) contain positive and negative ions, which are held together by the strong force of attraction between particles with opposite charges. When one of these solids dissolves in water, the ions that form the solid are released into solution, where they become associated with the polar solvent molecules.
We can generally assume that salts dissociate into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution. There are a number of patterns in the data obtained from measuring the solubility of different salts. These patterns form the basis for the rules outlined in the table below, which can guide predictions of whether a given salt will dissolve in water. These rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. A salt is soluble if it dissolves in water to give a solution with a concentration of at...

Good report
this was useful to me!
it's generally well done.
0 out of 0 people found this comment useful.