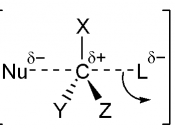Nor Amirah Farhana Nawawi Organic Chemistry Lab Report Bo Shen
Title: Nucleophillic Substitution Reaction
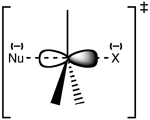
Introduction: This lab experimented on the reactions of the chemicals that undergoes SN1 or SN2 reaction. Nine different compounds were given to be examined with two reagents - NaI in acetone and AgNO3 in ethanol. The SN1 reaction happens in AgNO3 in ethanol reagents, and SN2 reaction is in NaI in acetone.
Procedure: Following the lab manual.
Results:
compound | NaI /acetone (SN2) | AgNO3/ethanol (SN1) |
Bromobenzene | NR | NR |
Bromocyclopentane | X | 2 sec |
Bromocyclohexane | NR | 4 sec |
2-bromobutane | 5 min | 2 sec |
2-chlorobutane | NR | X |
Chloroacetone | 7 sec | X |
1-chlorobutane | X | X |
t-butyl chloride | NR | 6 sec |
Benzyl chloride | 3 min | X |
Conclusions and discussion
Bromobenzene undergoes no reaction for both SN1 and SN2. This is because bromobenzene is very stable, and contains allylic and vinyllic bromine, which is also very stable, and cannot be a good nucleophile.
Bromocyclopentane reacts under SN1 and SN2, but it shows a faster reaction in AgNO3/ethanol reagent, that is SN1. This is because bromocyclopentane is secondary bromine, and have bigger steric strain, since it is a cyclic compound. The bigger steric in a molecule, the harder it is for the nucleophile to attack the leaving group (-Br) from the opposite sides, therefore, SN2 reaction is slower than SN1 for bromocyclopentane. From this reaction, precipitation occurs to give out AgBr in ethanol and NaBr in acetone.
Bromocyclohexane, on the other hand, shows no reaction in SN2 reagent, but almost an immediate reaction in SN1 reagent. The reasons are very much the same with bromocyclopentane above - steric strain. As bromocyclohexane is bigger than that of bromocyclopentane, the steric in bromocyclohexane is too much for the nucleophillic attack from the opposite side of the leaving Bromine. In the end, only SN1 reaction could...