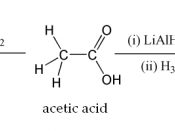
Question:
What is the molar concentration of acetic acid in a sample of vinegar?
Prediction:
The manufacture of claims on the label that the vinegar contains 5.0% acetic acid, which translates into a 0.87 mol/L concentration of acetic acid. The concentration of acetic acid in the vinegar sample should be the same.
Purpose:
If we add acid solution to basic solution to produce water and salt this activity is called titration. It involves carefully adding one solution to another until chemically equivalent amounts react. Vinegar is a solution of a weak acid in water. This acid will react with the base sodium hydroxide in a 1:1 molar ratio. If a solution of NaOH of known concentration will used titrate vinegar, it is possible to determine the amount of acetic acid. The main three things from this experiment are: to determine the concentration of vinegar, to verify the description on the product labels, practice the chemical testing technique of titration.
The balanced chemical equation for the neutralization of acetic acid with NaOH is:
Safety notes:
Wear safety glasses and if available. NaOH is corrosive to flesh and can cause blindness. Wash up spills immediately using large amounts of water. Rinse your hands under running water if you come in contact with the NaOH solution. Wash your hands upon completion of the lab.
Materials:
Lab apron eye protection
NaOH(aq) vinegar
Phenolphthalein wash bottle of pure water
Two 100mL or 150mL 250mL beaker
100mL volumetric flask with stopper 50mL buret
10mL volumetric pipet pipet bulb
ring stand buret clamp
stirring rod small funnel
two 250mL Erlenmeyer flasks meniscus finder
Procedure:
1. 10mL of vinegar was obtained in a clean, dry 100mL beaker.
2. One 10.00mL portion was pipeted into a clean 100mL volumetric flask and dilute to the mark.
3. The mixture...