Objective:
To draw a titration
Do calculations to figure out the theoretical pH of each titration
Theory:
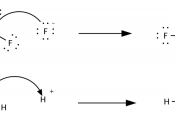
Every liquid has either acidic or basic traits. A chemist named Svante Arrhenius created a new definition for acids and bases in 1887. He saw that when molecules were placed into water, they sometimes broke down to relase an H+ (hydrogen) ion. Other times, OH- ions are released. Arrhenius also stated that when a hydrogen ion is released, the solution becomes acidic. And if a hydrozide ion is released, the solution beomes basic. Another definition fo acids and bases come from the chemist Bronsted. He developed the Bronsted acid-base theory, He argued that all acid-base reactions ivolve the transfer of an H+ ion, or proton. According to this theory, an acid is a "proton donor" and a base is a "proon acceptor". In 1932, G. N. Lewis suggested another definition for acids and bases.
In the Lewis theory of acid-base reactions, bases donated pairs of electons and acidsa accept pairs of electrongs. A Lewis acid is any substance that can accepet a piar of nonbonding lectrson. Therefore,a Lewis acid is an electron-pair accepto. Ont eh other hand, a Lewis base is any substance that can donate a pair of nonbonding electrons. This makes a Lewis base an electron-pair donor. Ofther, acids are divided into categories susch as "strong" and "weak". One measure of the strength of an acid is the acid dissociation equilibrium constant, or the Ka, for that specific acid. If the Ka is realtively large,t eh it is a srong acid. For example, HCl has a Ka of 1 x 10^3. The high Ka makes the HCl a strong acid. Reversely, when the value of Ka is small, then the acid is weak. For example, acetic acid has a Ka of...