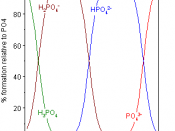
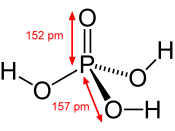
Vibrational data on adsorbed anions contribute to the description of the electrochemical double layer as well as add to the physiochemical properties of the electrified surface. In this study, phosphoric acid is one of the best systems to monitor. In a previous study, it was discovered that the nature and geometry of the phosphate species is dependant upon applied potential. Using cyclic voltammetry and in situ FTIR spectroscopy, the adsorption of phosphate species on a single crystal platinum was studied. The pH range was between 1.03 and 12.7. With an increased potential on polycrystalline platinum, the phosphate species became more protonated.
From the spectroscopy viewpoint, it may seem difficult to investigate the phosphate adsorption because of the polyprotic nature of phosphoric acid. This would make several anionic species in the adsorption process. It is therefore very important to use the appropriate electrolyte solutions. So in this article, the phosphate species is monitored as an adsorption on Pt(111) and Pt(100) in acid media.
The in situ FTIR experiment was done a Digilab FTS-40 IR spectrometer. The Pt(111) and Pt(100) crystal electrodes were prepared, pretreated by flame, and protected by a drop of deaerated Millipore MilliQ water as it was transferred to the spectroelectrochemical cell. The counter electrode was a platinum flat ring, and a hydrogen charged Pd net was the reference electrode. Then mixtures of HF and KF were made. The mixture of 0.69M HF and 0.5M KF were used as the base electrolyte with a pH of 2.8. A 7.3M HF solution at a pH 0.23 was the base electrolyte. By alternating the potential after each 1000 scans, the in situ FTIR spectrum was obtained. The reference potential was set at 0.03V vs. Pd/H2. This procedure results in positive-going bands (due to the loss of solution species) and negative-going bands...