For all living organisms, water is the most important substance on Earth. If there would be no water on Earth, there would be no life on our planet, since life has always been closely connected to water.
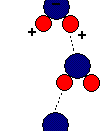
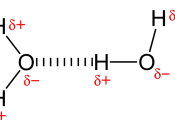
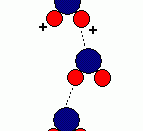
Due to its structure, water has many characteristics, which make it unique. Because of its molecular structure, water is a polar molecule. The unequal distribution of charges between the poles of the water molecule defines the its behaviour near charged objects.
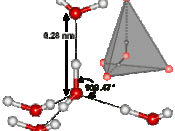
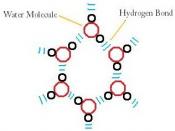
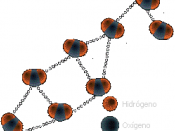
As a result of hydrogen bonding, water molecules tend to stick to each other, which holds the structure together (cohesion). This gives water the specific shape of edge, elevation, rate of evaporation, and surface tension.
Due to hydrogen bonding, water has a high heat capacity, which makes it different from many substances. This means you need more energy in order to change the temperature of water, that most of the other substances.
The hydrogen bonding is also responsible for the specific density and its solvent properties.
Too short to be of any use.
I honestly don't remember doing anything this short when I was in high school its more like a awnser to a question out of a text book.
1 out of 1 people found this comment useful.