Experiment: Galvanic CellsPurpose: to perform an investigation to measure the difference in electrical potential of differentcombinations of metals in an electrolyte solution using standard conditions. Also, to account forthe difference in potential between different combinations of metals and discuss the differencein the results from the calculated theoretical values.
Hypothesis: When various metals are tested with the same concentration of solution we will find that the further apart the metals are in the Activity series, the higher the cell voltage (potentialdifference). Positive ions (cations) will move to the cathode and the negative ions (anions)will move to the anode.
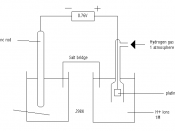
Plan: Collect the required materials and equipment. Clean two beakers. Half fill these two beakers with50mL each of the corresponding electrolyte solution to the metal being tested. Fold a piece of filterpaper into a strip. Dip this completely into potassium nitrate solution and squeeze out any extrasolution so that the paper does not drip.
This is the salt bridge used in this experiment. Fold the saltbridge in half and dip each end of it into each electrolyte solution so that the ends are submerged inthe electrolyte solution. Collect the two electrodes that the two electrolyte solutions corresponds to.
Thoroughly clean each of these electrodes (or piece of metal in the case of magnesium) with emerypaper. Place each electrode into its corresponding electrolyte solution. Attach, to the electrodes, apiece of wire each using crocodile clips; then attach each of these two wires into a multimeter tomeasure to voltage produced by the two half cells. Record the results in an appropriate manner.
Repeat the experiment using various combinations of electrodes and their corresponding electrolytesolutions, cleaning the beakers between each test. Repeat the whole experiment to make it morereliable.
Equipment: - 1ÃÂ voltmeter- 2 ÃÂ leads- 2 ÃÂ crocodile clips- 2 ÃÂ 50ml beakers- 30 ÃÂ...

![Reactions of Zinc(II)-nitrate. From left to right: With (NH4)2S: Zn2+ - S2- -> ZnS(s) (white sulfide, here disperged in yellow (NH4)2S With NaOH: Zn2+ + 2OH- -> Zn(OH)2; Zn(OH)2 + 2OH- [Zn(OH)4]2- (The white precipitation dissolves in surplus as visible i](https://s.writework.com/uploads/10/108792/reactions-zinc-ii-nitrate-left-right-nh4-2s-zn2-s2-zns-s-whi-thumb.jpg)