tionIntroduction:In order to produce an alkyl arene, aromatic rings usually undergo the Friedel-Crafts alkylation. This is usually done in the presence of an alkyl halide with a Lewis acid catalyst. As limitations for this reaction to take place, the arene should be unsubstituted or should have an activating group attached. Such activating groups include: -OH, -OCH3, and -CH3 which provide extra resonance stabilization. Deactivating groups are electron withdrawing groups such as ÃÂNO2. These withdrawing groups fail most of the time to carry out this type of reaction due to the lack of resonance stabilization.
In this reaction the arene p-xylene is the starting aromatic compound. In this case the p-xylene is more reactive than the starting material, n-propyl chloride and aluminum chloride, so multiple substitutions can take place. The p-xylene is present in excess to prevent multiple alkylations of the reaction to take place.
Rearrangements such as carbocation rearrangements and methyl/hydride shifts can result in different alkyl arenes as products.
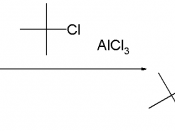
The alkyl halides, with an exception to methyl and ethyl, undergo these rearrangements. Usually, under the Friedel-Crafts alkylation conditions, primary alkyl halides rearrange to either secondary or tertiary carbocations that are much more stable. The reactant in this experiment is n-propyl chloride. The products in this experiment from the p-xylene are the unrearranged n-propyl group attached to the p-xylene (1,4-Dimethyl-2-n-propylbenzene) and the rearranged isopropyl group attached to the p-xylene (1,4-Dimethyl-2-isopropylbenzene). The aluminum chloride is the Lewis acid catalyst in the reaction and combines with the chloride ion of the n-propyl chloride to form the Friedel-Crafts complex.
Gas chromatography will be used to decide the quantitative product distribution of the resulting compounds.
The purpose of this experiment is to carry out the Friedel-Crafts alkylation of p-xylene. Also the rearrangement of the primary alkyl group, n-propyl chloride, should be displayed. Finally the percent composition of each product should be determined by the gas chromatography results. It is expected that the 1,4-Dimethyl-2-isopropylbenzene will be formed at a greater percentage due to the more stable and thus faster isopropyl cation. The hydride shift speeds up the process of this Friedel-Crafts alkylation reaction.
Reaction Equation:Experimental Section:A 25 mL round bottom flask was weighed, recorded, and 7.4 mL of dry p-xylene was added. The weight of the flask was then reweighed and the weight of the p-xylene was determined by subtraction. The round bottom flask weighed 23.68 g. The round bottom flask with the p-xylene weighed 32.25 g, so therefore the p-xylene weighed 8.57 g to start (.081 moles). A magnetic spin bar was added to the flask. A Claisen adaptor was attached to the round bottom flask, with a rubber cap at the one opening and a calcium chloride drying tube attached to the other end. Paying carful attention 0.31 g of anhydrous aluminum chloride was weighed out and added to the reaction flask quickly. The aluminum chloride was added to the reaction flask under the hood very quickly because of its reactive nature with the atmospheric moisture. A conical vial was weighed and 2.7 mL of 1-chloropropane of the source Aldrich was added. The vial was then reweighed and the amount of 1-chloropropane was calculated. The initial weight of the vial was 26.78 g and the weight of the vial and the 1-chloropropane was 28.99 g. By subtraction, the 1-chloropropane was weighed and recorded as 2.21 g (.028 moles). The 1-chloropropane was then added to the reaction flask drop wise with a syringe. The reaction flask was then left sit for an hour in contact with the stir plate at room temperature. The mixture in the reaction flask during this hour was an orange/yellow color. After the period of an hour, 8.0 mL of water was added to the reaction flask. The addition of the water resulted in white smoke. The flask was kept on the stir plate until the aluminum chloride was completely consumed. The mixture was then transferred to a 125 mL separatory funnel and the bottom aqueous layer was discarded. The top layer was a milky white color while the bottom layer was clearer but not completely translucent. This process was completed again. Instead 5% aqueous sodium bicarbonate solution was added to the seperatory funnel. This solution was added to the funnel in order to get rid of any more water present. The same separation appearance as the step before was seen again. This process of separation was done once more with the addition of 6.0 mL of water added to the separatory funnel. After the lower aqueous layer was discarded, the rest of the solution was then transferred to a 25-mL Erlenmeyer flask. The flask was let sit for ten minutes with occasional swirling, after 2.0 g of anhydrous sodium sulfate was added. The remaining solution was then pipetted into a vial and a gas chromatogram of the product was obtained and analyzed. The same procedure was followed by a fellow student with 2-chloropropane as the alkyl halide and the gas chromatogram was obtained and analyzed.
GC results: (GC of starting material attached)ComponentRT (min)%AreaStandard 1-chloropopane0.55100.00Standard 2-chloropropane0.4641.650.5258.35Standard p-xylene0.591.071.0198.93Product w/ 1-Chloropropane Product w/ 2-chloropropaneThe data obtained from GC analysis of the products from the addition of 1-chloropropane were as follows:ComponentRT (min)%AreaComponent 10.610.73Component 21.0062.95Component 32.1315.31Component 42.3220.996The data obtained from GC analysis of the products from the addition of 2-chloropropane was as follows:ComponentRT (min)%AreaComponent 11.0363.38Component 22.1236.62The Gas Chromatogram showed different results for both of the different reactants used. The GC for the 2-chloropropane showed the retention timed of the reactant, p-xylene, and the 1,4-Dimethyl-2-n-propylbenzene product. The GC for the 1-chloropropane that was used gave the retention times of the reactant, p-xylene, the 1-chloropropane reactant and the two possible products of 1,4-Dimethyl-2-n-propylbenzene and 1,4-Dimethyl-2- isopropylbenzene.
Discussion:The gas chromatograms show conclusive results. Comparing to the parameters of the p-xylene, 1-chloropropane, and 2-chloropropane the chromatograms of the resulting products were clear. For the GC of the reaction done with the 2-chloropropane there was a retention time of 1.03 for the p-xylene that matched up with the retention time of 1.01 of the given p-xylene. Also the retention time of 2.12 shown represents the 1,4-Dimethyl-2-isopropylbenzene that didnÃÂt have to undergo carbocation rearrangements with the 2-chloropropane as a reactant.
The 1-chloropropane reactant chromatogram showed a retention time of 0.61 for the 1-chloropropane matching the 0.55 retention time of the given. The 1.00 retention time equals that of the given GC for the p-xylene. The two large retention times of 2.13 and 2.32 represent both of the products formed in this reaction with the 1-chloropropane as a reactant. Both of the possible products showed up on this GC because of the carbocation rearrangement to produce more stability. The primary alkyl halide of 1-chloropropane underwent a carbocation rearrangement to speed up the reaction. Both the 1,4-Dimethyl-2-n-propylbenzene and the 1,4-Dimethyl-2-isopropylbenzene are represented in this GC. The prediction of rearrangement was proven.
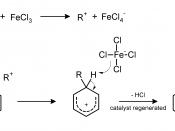
Mechanism:Once generated by the reaction of n-propyl chloride and aluminum chloride, the resulting Friedel-Crafts complex can undergo a hydride shift, breaking the carbon-chlorine bond and forming an isopropyl cation. This cation will eventually be attacked by a double bond from p-xylene. This forms a carbocation in the arene. The double bond is then reformed when the chlorine from the tetrachloroaluminate ion attacks the hydrogen on the carbon of the isopropyl group.
Conclusion:Through experimentation it was clear that the use of a different isomer of the reactant gave way to different products as seen with the help of the Gas Chromatogram. The process of Friedel-Crafts alkylation was carried out properly and the products of this reaction were well defined by the Gas Chromatogram results. The 1-chloropropane reactant, with electrophilic aromatic substitution gave way to the 1,4-Dimethyl-2-n-propylbenzene product and the rearranged 1,4-Dimethyl-2-isopropylbenzene. While the already stable carbocation once formed yielded the more stable product of 1,4-Dimethyl-2-isopropylbenzene. The rearrangement of the n-propyl chloride cation was displayed when the 1-chloropropane was used to form a more stable secondary carbocation product.
References:MacKay, Elizabethtown College, Department of Chemistry and Biochemistry, A Friedel Crafts Alkylation. Modified 8/21/09OÃÂNeil, Maryadele J. The Merck Index. 14th Ed. Merck & Co., Inc.: NJ, 2006.