�PAGE � �PAGE �9�
A general overview of chaperone proteins, and the purpose, function and behaviour at a molecular level of the Hsp70 family of chaperone proteins
Ava Rafii
Mrs. T. Davidson-Grossman
January 8, 2010
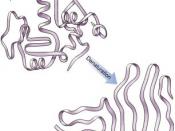
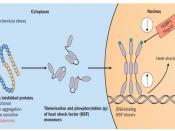
Chaperone proteins are a class of cellular proteins situated all over the cell, in a variety of cellular compartments (Welch & Brown, 1996), whose basic role is to ensure the correct folding of certain polypeptide chains and their assembly into oligometric structures (Ellis, 1987). They can more specifically be defined as "â¦proteins that bind to and stabilize an otherwise unstable conformer of another protein - and by controlled binding and release, facilitate its correct fate in vivo, be it folding, oligometric assembly, transport to a particular sub cellular compartment, or disposal by degradationâ¦"; they increase yield, but do not increase rate of folding (Hartl, 1996). Chaperone proteins work as molecular "chaperones" to disrupt "improper" interactions - such as even a fleeting exposure of hydrophobic or charged surfaces, which would cause aggregation - that may occur despite their chaperonage (Ellis, 1987). They are also able to disassemble aggregated structures (Ellis, 1987).
The name "chaperone proteins" was first used to describe nucleoplasmin, as nucleoplasmins chaperone histones by shielding their positive charges; however, the name now covers more generally all bodily proteins (be they viral, animal or plant) that do not form part of the final structure; they need not even possess steric information specifying assembly (Ellis, 1987).
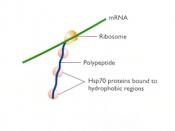
Chaperone proteins interact with nascent proteins throughout protein synthesis; however, these interactions are transient, since once the protein is properly folded it is no longer viewed as a substrate by the chaperone proteins (Welch & Brown, 1996). Proteins that are unable to become thermodynamically stable as well as properly folded -...