An Investigation of Photosynthetic Electron Transport of Chloroplasts from Silverbeet Leaves
Introduction
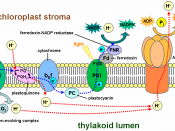
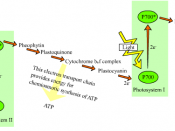
Photosynthesis is the process by which plants use the energy from sunlight to produce sugar, which cellular respiration converts into ATP, the "fuel" used by all living things. The conversion of unusable sunlight energy into usable chemical energy is associated with the actions of the green pigment, chlorophyll. Light Dependent Reactions are the initial stage of photosynthesis, in which solar energy is converted into potential energy. This reaction produces oxygen gas and converts ADP and NADP+ into ATP and NADPH.
Among all living organisms on planet earth, only plants are capable of producing their own food and deriving energy from it. By producing energy, the plants supply all the necessary nutrients and energy directly/indirectly to the other living creatures. The production of this energy is possible through photosynthesis.
The aim of this practical is to investigate the effects of different treatments on photosynthetic electron transport by using isolated chloroplasts from silverbeet leaves.
As photosynthesis proceeds, any electrons produced will reduce the dye DCPIP to its colourless form, so a rapid decrease in dye colour will indicate a rapid rate of photosynthetic electron transport. Given this, I predict that placing the reaction in the dark will produce no change in colour as well as the treatment of adding DCMU, as it is an inhibitor of photosynthesis, and the treatment of boiling. I also predict that the treatment using only red wavelengths of light will proceed at a more rapid rate of photosynthetic electron transport than the treatment using only green wavelengths of light, resulting in a rapid decrease of dye colour.
Method
Table 1. Experimental design for the electron transport experiment.
TREATMENT | ||||||||
BLANK 1 | DARK 2 | LIGHT 3 | BOILED 4 | DCMU 5 | RED 6 | GREEN 7 | ||
A | chloroplast suspension (ml) | 1.5 | 1.5 | 1.5 | - | 1.5 | 1.5 | 1.5 |
B | buffered sucrose (ml) | 5.5 | 5.3 | 5.3 | 5.3 | 5.2 | 5.3 | 5.3 |
C | boiled chloroplast suspension (ml) | - | - | - | 1.5 | - | - | - |
D | 0.01 M DCMU (ml) | - | - | - | - | 0.10 | - | - |
E | DCPIP (ml) (add this last) | - | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
Seven spectrophotometer tubes were numbered and solutions A-D were added according to the volumes shown in Table 1. Tube 1 was capped and inverted several times. The absorbance was calibrated using Tube 1, which contained chloroplasts and sucrose only, as the blank, to ensure that any changes in colour for the other treatments could be attributed to the rate of the dye DCPIP. At time zero (mins), absorbance was recorded for all treatments immediately after addition of the dye DCPIP and mixing of contents. Immediately following the time zero reading, tube 2 was wrapped in foil and tubes 6 and 7 were placed into larger tubes covered in red and green cellophane respectively. Tubes 1-5 were also placed into larger tubes. All tubes were then placed horizontally on ice, under lights. At fifteen minute intervals, readings of absorbance were taken for all treatments, except for the dark tube which was kept wrapped in foil for 60 minutes, after which its absorbance was measured.
Results
There was a very slight decrease in the absorbance where the reaction mix was kept in the dark (Tube 2) (Fig.1) while the reaction mix where DCMU was added (Tube 5) (Fig.1) and where it was boiled (Tube 4) (Fig.1) had also very little change. On the other hand, there was a large decrease for the reaction mix that was kept in the light (Tube 3) (Fig. 1) and for the reaction mixes wrapped in red and green cellophane (Tubes 6 & 7) (Fig.1).
Discussion
My results did not concur with my predictions for Tube 2 (Dark), as I expected it to maintain the same absorbance throughout however it did for Tube 3 (Light) where I predicted that photosynthesis would occur due to exposure to the light. This is due to photosynthesis requiring the direct energy of light for it to occur as it is a process of converting light energy to chemical energy. The process of photosynthesis takes place in the chloroplasts, specifically the chlorophyll, so as Tube 2 was wrapped in foil, the chloroplast could not absorb any light for photosynthesis to occur. However, in the results, the absorbance decreased suggesting that when removing the foil to measure the tube in the spectrophotometer, it was exposed to light and causing rate of photosynthesis to occur.
In Tube 4 (Boiled), as the chloroplast has been boiled, the enzymes begin to lose their shape (become denatured) because by increasing the temperature, we are increasing the kinetic energy and the bonds holding the enzymes together are broken and therefore, the active sites are destroyed. This means they are unable to function properly and the rate of photosynthesis decreases again. Also, at higher temperatures the stomata close to prevent water loss. This also stops gas exchange which slows photosynthesis even further. I predicted that there will not be a decrease in absorbance (i.e change in colour) however, it turns out there was a slight decrease.
For Tube 5 (DCMU), I predicted that it would not decrease due to DCMU being a very specific and sensitive inhibitor of photosynthesis. DCMU blocks the passage of electrons from the primary acceptor of photosystem II to plastoquinone (Pq). This interrupts the photosynthetic electron transport chain in photosynthesis and therefore, blocks the ability of the plant to turn light energy into chemical energy (ATP and reductant potential). However, the results show that the DCMU did in fact, decrease in absorbance. This is because the concentration of DCMU is somewhat lower than that of DCPIP so the electrons are only moderately inhibited from passing to Pq and then to DCPIP. Consequently, DCPIP continues to be reduced, but at a slower rate than without DCMU and thus showing that even with the addition of DCMU, the rate of photosynthesis and the rate of electron transport still occurs, but albeit rather slowly.
My results for Tubes 6 (Red) and Tubes 7 (Green) did concur with my predictions. Chloroplasts contain a green pigment called chlorophyll looks green because it absorbs red and blue light, making these colours unavailable to be seen by our eyes. It is the green light which is not absorbed that is reflected and can finally reach our eyes, making the chlorophyll appear green. For Tube 6, the red light was absorbed better by the chloroplast which allows for photosynthesis to occur. However, for Tube 7, the green light cannot be absorbed by the chloroplast and thus, cannot be used to do photosynthesis. This results in Tube 6 having a much rapid decrease in dye colour and indicating a rapid rate of photosynthetic electron transport.
References
Campbell, Reece, et al. (2011). Campbell Biology: Australia Version (9e). Pearson Australia.