Experiment Four: Organic Synthesis of Aspirin
Abstract
The purpose of this experiment is to synthesize a common organic product called acetylsalicylic acid (aspirin), and to become familiar with the optimum conditions needed for successful yields. Aspirin is produced from an acid catalyzed reaction between salicylic acid with acetic anhydride. The crystalline aspirin is synthesized and purified by recrystallization, although there is not a hundred percent yield due to sources of error.
Introduction
Aspirin is a medicine commonly found in households around the world. It also is one of the least expensive and most useful drugs in the market. A Chemist named Felix Hoffmann first synthesized aspirin, otherwise known as acetylsalicylic acid, in 1897 from salicylic acid.1
Salicylic acid is a naturally formed substance. For example, willow trees have been recognized since ancient times as a natural source. Salicylic acid is found mainly in the willow's leaves and bark. The pure acid possesses several useful medicinal properties.
It is an antipyretic (a fever reducer), an analgesic (a pain reducer), and an anti-inflammatory (a swelling reducer).
Unfortunately, pure salicylic acid makes for a particularly unpleasant medicine. The salicylic acid molecule contains two acidic functional groups, the phenol group and the carboxylic acid group. These groups make salicylic acid irritating because it burns the sensitive linings of the mouth, throat, esophagus, and stomach. 2
These harsh qualities are alleviated by replacing the acidic hydrogens with less reactive groups of atoms: the acetyl group (COCH3). This results in acetylsalicylic acid or "aspirin".
Acetylsalicylic acid is synthesized in labs and does not occur naturally. It is produced from adding acetic anhydride to salicylic acid in the presence of sulfuric acid (H2SO4), an acid catalyst: (Diagram from 1)
The impurities left over (unreacted salicylic acid, acetic acid and sulfuric acid) are removed by the process of...
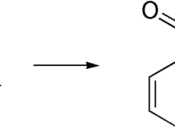
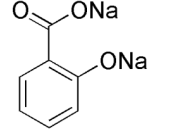
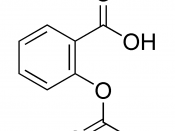
Acetyalation Catalysts
I found the following order of reactivity for four catalysts in the synthesis of aspirin. I am not sure if this order of activity is accurate, however.
Anhydrous sodium acetate < Boron trifluoride etherate < Pyridine < Concentrated sulfuric acid
I believe the H2SO4, sulfuric acid, is the best overall catalyst. I think it's used commercially (good and cheap).
1 out of 1 people found this comment useful.