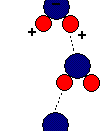
Hydride is the name given to any hydrogen compound. There are three types of hydrides: ionic hydrides, covalent hydrides and transitional metal hydrides. Ionic hydrides consist of hydrogen and an electropositive metal. The hydrogen atoms form ionic bonds with an electropositive metal by acquiring an electron from the metal to complete its S-shell. Covalent hydrides however consist of a covalent bond between hydrogen atoms and electronegative metals (generally groups 4-7). In transitional metal hydrides, the type of bonding varies between transition metals and the hydrogen atoms depending on certain factors: temperature, pressure and electric current.
When molecules change phases from liquid to gas, enough energy has to be applied to break the bonds between the molecules. This energy is known as kinetic energy. Since temperature is a way of measuring kinetic energy, boiling points would have to increase as kinetic energy increases. Therefore stronger bonds that require more energy to break would raise the boiling point as well.
Van der Waals forces originate between the polar charges particles of adjacent molecules. These intermolecular bonds may also be referred to as London dispersion forces. Their strength is dependant on the molecule's molecular size and shape. The bigger, longer and thinner the molecule, the stronger the van der Waals forces.
From the graph above we can notice three trends:
The lowest boiling points in all the periods are from group IV and all the highest are from group VI. On the other hand, the boiling points of groups V and VII cross over such that group seven has higher boiling points in periods 2 and 3, while group V has higher boiling points in periods 4, 5 and 6.
The boiling points of each group tend to increase with the exception of the boiling points of elements from period 2.
The...