Atomic Number - 34 Atomic mass- 78.96
FAMILY Group 16 Chalcogen
Physical properties
Selenium occurs in numerous allotropic forms, but is typically in an amorphous or crystalline structure. Selenium in an amorphous form is either a brick -red powder or in a black vitreous form.
These arrangements of selenium do not have definite melting points. However, they progressively become softer when they are heated converting to grey selenium at 180 ðC. This grey selenium has a 'hexagonal crystal lattice consisting of helical polymeric chains and is the most stable and dense form of selenium' (Calinpop 2012)
Two of the main physical features of selenium (in particular grey selenium) is
that it's a semiconductor, which allows it to conduct electricity and also a photoconductor ,changing light energy into electrical energy (Chemistryexplained 2012).
Chemical properties
Selenium compounds typically exist in the oxidation states âÂÂ2, +2, +4, and +6. Selenium is a moderately reactive element combining with hydrogen, chlorine, fluorine, and bromine (Chemistryexplained 2012).
It reacts with sulfuric and nitric acids and also forms compounds called selenides when reacted with a number of metals. An example is magnesium selenide (MgSe).
Two oxides are formed from selenium including: selenium dioxide (SeO2) and selenium trioxide (SeO3).
In its elemental form, selenium is fairly nontoxic. However, hydrogen selenide (H2Se) and other forms of selenium are extremely toxic to humans. (Helmenstine 2012)
Main uses
The two main uses of selenium are in electronics and glass making. Both these account for 50-60% of all selenium produced per year. In glass making the addition of selenium can have two different effects. One way is that it can cancel out the green colour that arises from iron impurities that are common in most glass making or if desirable, it's able to add its own brick-red colour...
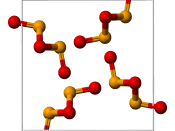
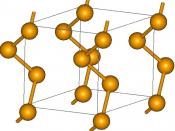
![Ball-and-stick model of an [SeO 2 ] n chain in crystalline selenium dioxide](https://s.writework.com/uploads/16/169136/ball-and-stick-model-seo-2-n-chain-crystalline-selenium-diox-thumb.png)