n. Physical properties-the result of hydrogen bonding
Studied in isolation, the water molecule is deceptively simple. Its tow hydrogen atoms are joined to the oxygen atom by single covalent bonds. Because oxygen is more electronegative than hydrogen, the electrons of the polar bonds spend more time closer to the oxygen atom. In other words, the bonds that hold together the atoms in a water molecule are polar covalent bonds, with the oxygen region of the molecule having a partial negative charge and the hydrogen having a partial positive charge. The water molecule, shaped something like a wide V, is a polar molecule, meaning that opposite ends of the molecule have opposite charges.
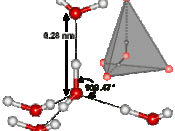
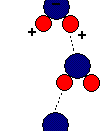
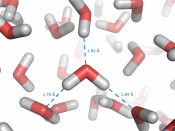
The anomalous properties of water arise from attractions between these polar molecules. The attraction is electrical; slightly positive hydrogen of one molecule is attracted to the slightly negative oxygen of a nearby molecule. The two molecules are thus held together by a hydrogen bond (FIGURE 1).
Each water molecule can form hydrogen bonds to maximum f four neighbors, and at any given moment, many of the molecules in a sample of liquid water are linked in this way. The extraordinary qualities of water are emergent properties resulting from the hydrogen bonding that orders molecules into a higher level of structural organization.
I will examine four of water properties that contribute to the fitness of Earth as an environment for life: waters cohesive behavior, its ability to stabilize temperature, its expansion upon freezing, and its versatility as a solvent.
1. Cohesion
Water molecules stick to each other as result of hydrogen bonding. When water is in its liquid form, its hydrogen bonds are very fragile, about 1/12 as strong as covalent bond. They form, break, and re-form with great frequency. Each hydrogen bond lasts only a few trillionths of...
Opinion
this is really very informative having great knowledge.
0 out of 0 people found this comment useful.