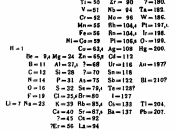Hydrogen is located in group 1, period 1. Hydrogen is element number 1 on the Periodic Table. Hydrogen was discovered by Cavendish in 1776. Lavoisier named hydrogen, which is the most abundant of all elements in the universe. The heavier elements were originally made from Hydrogen or from other elements that were originally made from Hydrogen. Hydrogen is estimated to make up more than 90% of all the atoms or three quarters of the mass of the universe. This element is found in the stars, and plays an important part in powering the universe through both the proton-proton reaction and carbon-nitrogen cycle; stellar hydrogen fusion processes that release massive amounts of energy by combining Hydrogen to form Helium. Although pure Hydrogen is a gas we find very little of it in our atmosphere. Hydrogen gas is so light that uncombined Hydrogen will gain enough velocity from collisions with other gases that they will quickly be ejected from the atmosphere.
On earth, hydrogen occurs chiefly in combination with oxygen in water, but it is also present in organic matter such as living plants, petroleum, coal, etc. It is present as the free element in the atmosphere, but only to the extent of less than 1 ppm by volume. The lightest of all gases, hydrogen combines with other elements sometimes explosively to form compounds.