Nobel Prize-caliber scientists concluded years ago that CFCs damage the ozone layer. So why is the debate still raging?
When the winners of the Nobel Prize for Chemistry appeared at the flower-filled Stockholm Concert Hall in December 1995 to receive their awards from King Carl XVI Gustaf, they were met by pickets. Why?
Because two of the three Nobelists, F. Sherwood Rowland and Mario Molina, were being rewarded for their pioneering work on the chemistry of ozone depletion. Their research showed that compounds called chlorofluorocarbons, or CFCs - once widely used as refrigerants and as propellants in spray cans - are responsible for the progressive thinning of the ozone layer, which shields us from the sun's ultraviolet radiation. The work of Rowland and Molina, along with that of other atmospheric scientists, has led to a worldwide phase-out of CFCs.
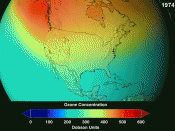
The worry about ozone arose in 1974, when researchers first calculated that depletion of the ozone would allow excess ultraviolet light from the sun to leak through and reach Earth's surface.
The impact of excess UV light could be increasingly dangerous, harming crops, wildlife and farm animals, and increasing the incidence of skin cancer in humans.
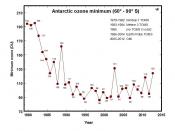
Meanwhile, ozone destruction has reached peak levels in recent years. The infamous ozone hole observed over Antarctica during the Southern Hemisphere's springtime has been opening earlier and lasting longer.
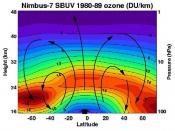
The major ozone loss, the hole, occurs every year inside a ring- shaped wind pattern called the polar vortex. In the zone of air between 10 and 13 miles high, the ozone was essentially being erased in springtime. Measurements soon showed that the flow of dangerous UV light had also increased at ground level directly under the ozone hole.
Researchers soon figured out that during the dark, cold winter months, airborne ice crystals tend to...