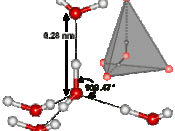
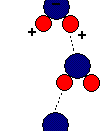
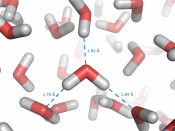
A water molecule consists of one atom oxygen with two atoms of hydrogen connected to it forming a ÃÂV.ÃÂ HOH is a polar molecule with means that charges unequally form around it. This distribution of charges creates a negative charge on the oxygen side and a negative charge on the side with the hydrogen atoms. This creates hydrogen bonding which gives water its unusual characteristics. Hydrogen bonds are weak temporary bonds that form when the positive part of one water molecule attracts a negative part of a different water molecule. Hydrogen bonding makes water have unusual properties that also make life possible on earth.
Hydrogen bonding causes water to be cohesive, which basically means it sticks to itself in liquid form. Hydrogen bonds connect one water molecule to the next, to the next, and so on. This is an important property because trees use it to get water and nutrients to the higher parts of the tree.
When water evaporates off of leaves water is being pulled from the trunk into where the water has evaporated from. This pulling force exerts all the way to the roots. The water brings along important nutrients also. This process is called capillary action. Surface tension is also formed because of cohesion. Water molecules along the surface of the water are not fully surrounded by other water molecules so they form more bonds along the edge of the water. This allows certain insects, such as the water strider to stride across the water.
WaterÃÂs high specific heat or its resilience to change temperature is also an important characteristic of water that makes water an unusual substance that makes life possible on earth. Because of this organisms are able to maintain a stable temperature. Is water didnÃÂt have a high specific heat than the water...
Improvement
Your essay seems to be simply a copy and paste from some website and sources, it doesn't seem too original. First of all, if you would like to make it better, you should write stronger and better thesis statement. Then you should add a conclusion to the essay as well.
0 out of 0 people found this comment useful.