A. In the late 1820's organic chemistry was the study of substances isolated from animals and plants that were thought to contain a "vital force." "Vitalism" was a theory that suggested that compounds found in living organisms were not constructable in laboratories. In those days, textbooks referred to the chemistry of such compounds as "animal chemistry" and "vegetable chemistry." It was a man named Fredrich Wohler who finally came along and proved the theory of vitalism wrong. What Fredrich tried to do was make ammonium cyanate by a double displacement reaction between silver cyanate and ammonium chloride. Since AgCl is insoluble, Fredrich filtered it off and then began the process of evaporating the rest of the water from the remaining solution. After the water evaporated, he was left with some white crystals. Upon analyzation of the crystals, he saw that they had no qualities that inorganic compounds usually possessed. The crystals were actually identical to crystals of urea, a compound found in urine.
Fredrich had completed an "ÃÂimpossible' task by making an organic compound out of inorganic materials. This experiment started the end of the "vitalism" theory.
Today organic chemistry is the study of chemistry of compounds that contain carbon. It should be kept in mind however that some carbon compounds are inorganic substances, such as carbon dioxide, carbon monoxide, hydrogen cyanide, carbonates, and cyanides.
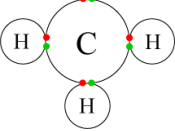
B. CH4- is an organic compound and would therefore possess almost pure covalent bonds since carbon and hydrogen have similar electronegativities as opposed to NaCl, which would have ionic bonds.
The melting point of CH-4 is low because it has weak dispersion forces, which are easy to overcome whereas NaCl has a very high melting point.
CH-4 is also insoluble in water because the action of the water molecules is not great enough to break...

Good
Commendable Job...Must use a proper bibliography / site sources.
Grammar seems consistent and so on.
Needs sources!!
0 out of 0 people found this comment useful.