In examining atomic structure though, we have to clarify this statement. An atom cannot be broken down further without changing the chemical nature of the substance. For example, if you have 1 ton, 1 gram or 1 atom of oxygen, all of these units have the same properties. We can break down the atom of oxygen into smaller particles, however, when we do the atom looses its chemical properties. For example, if you have 100 watches, or one watch, they all behave like watches and tell time. You can dismantle one of the watches: take the back off, take the batteries out, peer inside and pull things out. However, now the watch no longer behaves like a watch. So what does an atom look like inside?
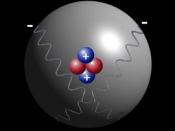
Atoms are made up of 3 types of particles electrons , protons and neutrons . These particles have different properties. Electrons are tiny, very light particles that have a negative electrical charge (-).
Protons are much larger and heavier than electrons and have the opposite charge, protons have a positive charge. Neutrons are large and heavy like protons, however neutrons have no electrical charge. Each atom is made up of a combination of these particles. Let's look at one type of atom:
A neutron walked into a bar and
asked how much for a drink.
The bartender replied,
"for you, no charge."
-Jaime - Internet Chemistry Jokes
The atom above, made up of one proton and one electron, is called hydrogen (the abbreviation for hydrogen is H). The proton and electron stay together because just like two magnets, the opposite electrical charges attract each other. What keeps the two from crashing into each other? The particles in an atom are not still. The electron is constantly spinning around the center of the atom (called...