Introduction
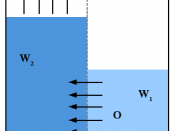
Osmosis is the movement of water from a less concentrated solution to a more concentrated solution through a partially permeable membrane.
Osmosis is affected by:
Water potential - This is the tendency of water to move from an area of higher concentration to lower concentration.
Temperature- Cells move faster at a high temperature therefore if there is a high temperature there is a higher rate of osmosis.
Pressure- Areas of different pressure have different water potentials.
Surface area- the larger the surface area, the more water can be absorbed into the cells, therefore altering the rate of osmosis.
In this experiment we wanted to show how the concentration of salt affects the rate of osmosis.
Hypothesis
As the concentration of salt solution is increased, the rate of osmosis increases.
Aim
My aim was to find out if the rate of osmosis increases with concentration of salt solution.
Independent variable
The concentration of salt solution placed in the dialysis tubes.
It is the independent variable because in every tube different amount of salt was put to make a difference.
Dependent variable
The weight of the dialysis tubes was the dependent variable. This was the variable that changed as a result of the concentration of the solution.
Control variable
The control variable is the amount of water that is used. This is because the same amount is used during the whole experiment and this is used to examine the rate of osmosis.
The other control variable was the time as it was constant in all concentrations.
Apparatus
A weighing scale
6 dialysis tubes
3 beakers
1 stopwatch
1 measuring cylinder
1 syringe
10 grams of salt
20 grams of salt
Distilled water
A ball of string
Method
We took a 20 cm dialysis tubing and soaked it in water to...