PURPOSE : In this particular laboratory experiment, we are going to determine the molar mass of an unknown gas, from the gas density. We are also going to use gas density, along with the kinetic molecular theory, to explain the behavior of gas filled balloons. Finally, we are going to demonstrate the effects of temperature and pressure changes of gasses.
PROCEDURE : We are using the experimental procedure as described in our laboratory manual.
University of Wisconsin - River Falls
Laboratory Manual
Chemistry 116
Experiment 116-8
Molar Mass From Gas Densities
Pages 81 & 82
There are no changes to this laboratory or from the indicated laboratory procedure.
MATERIALS and APPARATUS :
Part A-1: In this part of the experiment, we are going to observe three balloons filled each with one of three different gasses. So as part of our materials we need to have three balloons as well as the three gasses.
These balloons were already prepared for us and we kept underneath the fume hood for observation.
Helium Sulfur Exofluoride
He SF6
4.002602 g. 146.06 g.
Laboratory Stock Laboratory Stock
Ammonia
NH3
17.031 g.
Laboratory Stock
Part A-2-5c: During this part of the experiment, our materials and equipment consisted of one, clean, dry, 500mL plastic bottle with an airtight cover. Along with the bottle we need an electronic balance, a thermometer set up to measure the air temperature, as well as a barometer with a chart on how to interpret the readings. Finally we must have the six different gasses with which to do our experiment.
Distilled Water Helium
H2O He
33.00674 g. 4.002602 g.
Laboratory Stock Laboratory Stock
Oxygen Argon
O2 Ar
31.9988 g. 39.948 g.
Laboratory Stock Laboratory Stock
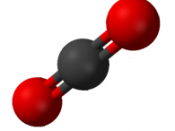
Carbon Dioxide Propane
CO2 CH3CH2CH3
44.0098 g. 44.09652 g.
Laboratory Stock Laboratory Stock
Natural Gas
CH4
16.04276...

Thoughts
Nice, and well explained. Very comprehensive, which is always good to see.
0 out of 0 people found this comment useful.