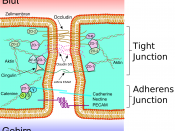
Anchoring junctions occur in two functionally different forms:Adherens junctions and desmosomes hold cells together and are formed bytransmembrane adhesion proteins that belong to the cadherin family.
Focal adhesions and hemidesmosomes bind cells to the extracellular matrixand are formed by transmembrane adhesion proteins of the integrin family.
On the intracellular side of the membrane, adherens junctions and focaladhesions serve as connection sites for actin filaments, while desmosomes andhemidesmosomes serve as connection sites for intermediate filaments. Adherens Junctions Connect Bundles of Actin Filaments from Cell to Cell. Adherens junctions occur in various forms. In many nonepithelial tissues, theytake the form of small punctate or streaklike attachments that indirectlyconnect the cortical actin filaments beneath the plasma membranes of twointeracting cells. But the prototypical examples of adherens junctions occur inepithelia, where they often form a continuous adhesion belt (or zonulaadherens) just below the tight junctions, encircling each of the interacting cellsin the sheet. The adhesion belts are directly apposed in adjacent epithelialcells, with the interacting plasma membranes held together by the cadherinsthat serve here as transmembrane adhesion proteins.
Within each cell, a contractile bundle of actin filaments lies adjacent to the adhesion belt, oriented parallel to the plasma membrane. The actin is attachedto this membrane through a set of intracellular anchor proteins, includingcatenins, vinculin, and a-actinin, which we consider later. The actin bundlesare thus linked, via the cadherins and anchor proteins, into an extensivetranscellular network. This network can contract with the help of myosin motor proteins and it is thought to help inmediating a fundamental process in animal morphogenesis the folding ofepithelial cell sheets into tubes and other related structures. The assembly of tight junctions between epithelial cells seems to require theprior formation of adherens junctions. Anti-cadherin antibodies that block theformation of adherens junctions, for example, also block the formation of tightjunctions.
Desmosomes Connect Intermediate Filaments from Cell toCellDesmosomes are buttonlike points of intercellular contact that rivet cellsTogether. Inside the cell, they serve as anchoring sites forropelike intermediate filaments, which form a structural framework of greattensile strength. Through desmosomes, the intermediatefilaments of adjacent cells are linked into a net that extends throughout themany cells of a tissue. The particular type of intermediate filaments attached tothe desmosomes depends on the cell type: they are keratin filaments in mostepithelial cells, for example, and desmin filaments in heart muscle cells.
The junction hasa dense cytoplasmic plaque composed of a complex of intracellular anchorproteins (plakoglobin and desmoplakin) that are responsible for connecting thecytoskeleton to the transmembrane adhesion proteins. These adhesion proteins(desmoglein and desmocollin), like those at an adherens junction, belong to thecadherin family. They interact through their extracellular domains to hold theadjacent plasma membranes together.
The importance of desmosome junctions is demonstrated by some forms of thepotentially fatal skin disease pemphigus. Affected individuals make antibodiesagainst one of their own desmosomal cadherin proteins. These antibodies bindto and disrupt the desmosomes that hold their skin epithelial cells(keratinocytes) together. This results in a severe blistering of the skin, withleakage of body fluids into the loosened epithelium.
Anchoring Junctions Formed by Integrins Bind Cells to theExtracellular Matrix: Focal Adhesions andHemidesmosomesSome anchoring junctions bind cells to the extracellular matrix rather than toother cells. The transmembrane adhesion proteins in these cell-matrixjunctions are integrins a large family of proteins distinct from the cadherins.
Focal adhesions enable cells to get a hold on the extracellular matrix through integrins that link intracellularly to actin filaments. In this way, muscle cells,for example, attach to their tendons at the myotendinous junction. Likewise,when cultured fibroblasts migrate on an artificial substratum coated withextracellular matrix molecules, they also grip the substratum at focaladhesions, where bundles of actin filaments terminate. At all such adhesions,the extracellular domains of transmembrane integrin proteins bind to a proteincomponent of the extracellular matrix, while their intracellular domains bindindirectly to bundles of actin filaments via the intracellular anchor proteinstalin, a-actinin, filamin, and vinculin.
Hemidesmosomes, or half-desmosomes, resemble desmosomesmorphologically and in connecting to intermediate filaments, and, likedesmosomes, they act as rivets to distribute tensile or shearing forces throughan epithelium. Instead of joining adjacent epithelial cells, however,hemidesmosomes connect the basal surface of an epithelial cell to theunderlying basal lamina. The extracellular domains of theintegrins that mediate the adhesion bind to a laminin protein (discussed later)in the basal lamina, while an intracellular domain binds via an anchor protein(plectin) to keratin intermediate filaments. Whereas the keratin filamentsassociated with desmosomes make lateral attachments to the desmosomalplaques many keratin filaments associated withhemidesmosomes have their ends buried in the plaque.
Although the terminology for the various anchoring junctions can beconfusing, the molecular principles (for vertebrates, at least) are relativelysimple. Integrins in the plasma membrane anchor a cell toextracellular matrix molecules; cadherin family members in the plasmamembrane anchor it to the plasma membrane of an adjacent cell. In both cases,there is an intracellular coupling to cytoskeletal filaments, either actinfilaments or intermediate filaments, depending on the types of intracellularanchor proteins involved.
Gap Junctions Allow Small Molecules to Pass Directly fromCell to CellWith the exception of a few terminally differentiated cells such as skeletalmuscle cells and blood cells, most cells in animal tissues are in communicationwith their neighbors via gap junctions. Each gap junction appears inconventional electron micrographs as a patch where the membranes of twoadjacent cells are separated by a uniform narrow gap of about 2 4 nm. Thegap is spanned by channel-forming proteins (connexins). The channels theyform (connexons) allow inorganic ions and other small water-solublemolecules to pass directly from the cytoplasm of one cell to the cytoplasm ofthe other, thereby coupling the cells both electrically and metabolically. Dyeinjectionexperiments suggest a maximal functional pore size for theconnecting channels of about 1.5 nm, implying that coupled cells share theirsmall molecules (such as inorganic ions, sugars, amino acids, nucleotides, vitamins, and the intracellular mediators cyclic AMP and inositoltrisphosphate) but not their macromolecules (proteins, nucleic acids, andpolysaccharides). This cell coupling has important functionalimplications, many of which are only beginning to be understood.
Evidence that gap junctions mediate electrical and chemical coupling has comefrom many experiments. When, for example, connexin mRNA is injected intoeither frog oocytes or gap-junction-deficient cultured cells, channels with theproperties expected of gap-junction channels can be demonstratedelectrophysiologically where pairs of injected cells make contact.
Biography:Cossart P, Boquet P, Normark S & Rappuoli R (eds) (2000) Cellular Microbiology. Washington: ASM Press.
Flint SJ, Enquist LW, Krug RM et al. (2000) Principles of Virology: Molecular Biology, Pathogenesis, and Control. Washington: ASM Press.
Janeway CA, Travers P, Walport M & Shlomchik M (2001) Immunobiology: The Immune System in Health and Disease, 5th edn. New York: Garland Science.
Salyers A & Whitt DD (1994) Bacterial Pathogenesis: A Molecular Approach. Washington: ASM Press.
Schaechter M, Engleberg NC, Isenstein BI & Medoff G (eds) (1988) Mechanisms of Microbial Disease. Philadelphia: Lippincott, Williams & Wilkins.
SL. Schmid. (1997). Clathrin-coated vesicle formation and protein sorting: an integrated process Annu. Rev. Biochem. 66: 511-548. (PubMed)T. Weber, BV. Zemelman, and JA. McNew, et al. (1998). SNAREpins: minimal machinery for membrane fusion Cell 92: 759-772. (PubMed)