The Biological Importance of Water
Water is a simple molecule, made of hydrogen atoms and oxygen atoms in the ratio of 2:1, yet it is fundamental to life. In active living cells, two-thirds, or often more, of the area is occupied by water, and two-thirds of the globe is covered in water. Water is therefore extremely abundant, and in biological terms it has great importance both inside cells, and externally, for example as a habitat. Water is the most abundant component of any organism. Humans are 60% water, and most organisms are 60-90% water. The water is found mainly in the protoplasm, and here it plays vital roles in many functions, for example in metabolism in all organisms, and photosynthesis and support in plants.
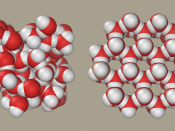
Water's importance can be explained referring to its unusual physical and chemical properties. It is the only substance which can be found naturally in all three states - solid (ice), liquid and gas (water vapour).
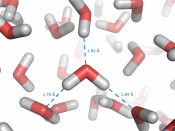
This due to the molecule being a polar molecule and so is able to form hydrogen bond between the molecules. The hydrogen atom has a slightly positive charge ,making it the positive pole and the oxygen atom has a slightly negative charge making it the negative pole. A bond can then form between the positive pole of one water molecule and the negative pole of another. This is called a hydrogen bond and is the strongest of intermolecular forces and a lot of these bonds form in liquid water, If these bonds did not exist, its boiling point would be in the region of -120oC, rather than the actual value of 100oC. Water is also very good at ionising substances due to its structure, and is therefore a good solvent.
Many different polar substances dissolve in water because of its polarity.

Hello
good
1 out of 1 people found this comment useful.