Kinetic Properties of Wheat Germ Phosphatase Results: 1. The final concentration of Acid Phosphatase enzyme in this reaction was 0.07 ìM, the final inhibitor concentration was 0.67 ìM. (see sample calculation one).
2. Table 1. Enzyme-Substrate Reaction (Uninhibited) Tube Concentration of PNPP (mM) Volume of 5.0mM PNPP (ml) Volume of dH2O (ml) A405 Velocity*(ìmol/min) 1 0 0 4.3 0 (blank) 0 2 0.05 0.05 4.25 0.148 0.0118 3 0.075 0.075 4.225 0.199 0.0159 4 0.113 0.113 4.187 0.231 0.0184 5 0.170 0.170 4.130 0.286 0.0228 6 0.255 0.255 4.045 0.337 0.0269 7 0.384 0.384 3.916 0.398 0.0318 8 0.578 0.578 3.722 0.414 0.0330 9 0.88 0.88 3.42 0.507 0.0405 10 1.32 1.32 2.98 0.578 0.0461 11 2.0 2.0 2.3 0.655 0.0523 12 3.0 3.0 1.3 0.708 0.0565 7? 0.384 0.384 3.916 0.324 0.0259 * See Sample Calculation #2. 7? is a rerun of tube #7.
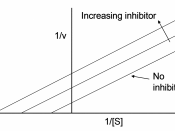
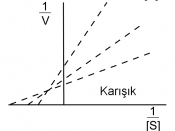
3. Table 2. Enzyme-Substrate Reaction (Inhibited) Tube Concentration of PNPP (mM) Volume of 5.0mM
PNPP (ml) Volume of 1.5mM K2HPO4 (ml) Volume of dH20 (ml) A405 Velocity(ìmol/min) 1 0 0 1 3.3 0 (blank) 0 2 0.05 0.05 1 3.25 0.002 0.0002 3 0.075 0.075 1 3.225 0.002 0.0002 4 0.11 0.11 1 3.19 0.023 0.0018 5 0.17 0.17 1 3.13 0.070 0.0056 6 0.26 0.26 1 3.04 0.081 0.0065 7 0.38 0.38 1 2.91 0.103 0.0082 8 0.58 0.58 1 2.72 0.116 0.0093 9 0.88 0.88 1 2.42 0.161 0.0128 10 1.32 1.32 1 1.98 0.226 0.0180 11 2.00 2.00 1 1.3 0.302 0.0241 12 3.00 3.00 1 0.3 0.418 0.0334 3. Graph one 4. Graph two 5. Table 3: Km and Vmax values Plot type Km uninhibited Km inhibited Vmax uninhibited Vmax inhibited MM* 0.28 4.00 0.72 0.95 LB 0.15 31.09 0.047 0.62 * See attached Kinetic printouts.
Sample Calculations 1. Enzyme: V1M1 = V2M2 (0.2ml)(2.5ìM Acid Phosphatase) = (7.5ml)(M2)...