Experiment 3
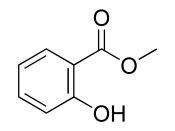
Hydrolysis of Methyl Salicylate
Aim
To produce salicylic acid via the hydrolysis of methyl salicylate under basic conditions.
Introduction
Methyl Salicylate is a colourless liquid at room temperature is a naturally occurring compound found in winter green oil used as a rubefacient in deep heating liniments and in small amounts as a flavouring agent at no more than 0.04%.[1]
Salicylic acid is a colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. It is best known for its use in anti-acne treatments. [ 2]
Reflux was a heating process whereby vapours from the reaction mixture were condensed and returned to their original flask. This ensure that the reaction could be maintained at the boiling point of the solvent while the solvent was not lost to the atmosphere. This was an important step in ensuring a higher yield of salicylic acid by ensuring that sodium salicylate, the compound formed after hydrolysis of NaOH was not boiled off and lost as vapour.
Suction filtration was used to collect the desired crystals for and in the recrystallisation procedure. Vacuum filtration occurs faster than gravity filtration by lowering the pressure in the flask, pulling the filtrate into the flask, speeding up the whole process of filtration. It ensured that crystals ere separated from water as much as possible, so that the crystals could dry faster and the melting points could be observed accurately in a shorter period of time.
Recrystallisation was carried out in order to purify the salicylic acid crystals as the first batch of crystals produced contained many contaminants such as Cl- from the acidification previously carried out, resulting in impure salicylic crystals. Hot water was added in minimal amounts to obtain a saturated solution...